发现新型 2,4,5-三取代嘧啶衍生物作为针对守门员突变体的强效、选择性 FGFR 抑制剂用于治疗 NSCLC
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
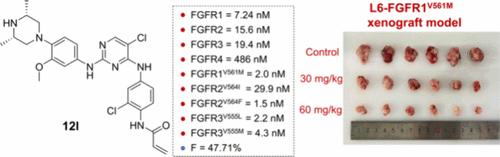
成纤维细胞生长因子受体(FGFRs)在调节癌细胞增殖、分化和迁移中起着至关重要的作用。然而,对FGFR抑制剂的获得性耐药的发展仍然是治疗非小细胞肺癌(NSCLC)的主要挑战,特别是由于看门人残基的突变。在这项研究中,我们报告了利用2,4,5-三取代嘧啶支架靶向FGFR1-3中看门人突变的一系列不可逆FGFR抑制剂的发现。经过合理设计、构效关系优化和药动学评价,化合物ng 12l成为具有前景的候选药物。它在体外显示出对FGFR1-3看门人突变的有效抑制以及良好的药代动力学特性。使用L6-FGFR1V561M/F细胞的实验证实了12l靶向FGFR1守门人突变的有效性。此外,在使用H1581和L6-FGFR1V561M细胞的异种移植模型中,12l表现出强大的抗肿瘤活性,毒性很小。这些发现表明12l是一种很有前景的治疗药物,可以克服NSCLC中守门人介导的耐药。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery of Novel 2,4,5-Trisubstituted Pyrimidine Derivatives as Potent and Selective FGFR Inhibitors against Gatekeeper Mutants for the Treatment of NSCLC
Fibroblast growth factor receptors (FGFRs) play a critical role in the regulation of cancer cell proliferation, differentiation, and migration. However, the development of acquired resistance to FGFR inhibitors remains a major challenge in treating non-small cell lung cancer (NSCLC), particularly due to mutations at the gatekeeper residue. In this study, we report the discovery of a series of irreversible FGFR inhibitors targeting gatekeeper mutations in FGFR1–3, utilizing a 2,4,5-trisubstituted pyrimidine scaffold. Through rational design, structure–activity relationship optimization, and pharmacokinetic evaluation, compound ng 12l emerged as a promising candidate. It demonstrated potent inhibition of FGFR1–3 gatekeeper mutations in vitro along with favorable pharmacokinetic properties. The efficacy of 12l in targeting FGFR1 gatekeeper mutations was confirmed in assays using L6-FGFR1V561M/F cells. Furthermore, in xenograft models using both H1581 and L6-FGFR1V561M cells, 12l exhibited robust anti-tumor activity with minimal toxicity. These findings position 12l as a promising therapeutic agent for overcoming gatekeeper-mediated resistance in NSCLC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


