机器学习辅助识别粪便细胞外囊泡 microRNA 标志,用于无创检测结直肠癌
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
结直肠癌(CRC)仍然是对人类健康的巨大威胁,其诊断存在相当大的挑战,特别是在恶性肿瘤的早期阶段。在这项研究中,我们阐明了粪便细胞外囊泡microRNA特征(FEVOR)可以作为有效的无创结直肠癌生物标志物。FEVOR首先通过miRNA测序揭示,随后构建了基于CRISPR/ cas13的检测平台,在不同的临床队列中询问FEVOR表达。随后,机器学习驱动的模型在CRC诊断、预测和早期预警系统领域得到了发展。在38例CRC患者队列中,我们的诊断模型准确率达到了97.4%(37/38),成功识别了38例CRC患者中的37例。这一表现明显超过了两种临床建立的生物标志物CEA和CA19-9的诊断效率,它们的准确率分别仅为26.3%(10/38)和7.9%(3/38)。我们还检测了FEVOR在几个结直肠癌患者手术前后以及结直肠腺瘤(CA)患者中的表达水平。令人印象深刻的是,结果表明FEVOR可以作为CRC的一个可靠的预后指标和CA的潜在预测因子。本研究旨在利用FEVOR的预测能力来提高CRC管理模式的准确性和有效性。我们设想这些发现将推动结直肠癌的基础和临床前研究以及临床研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
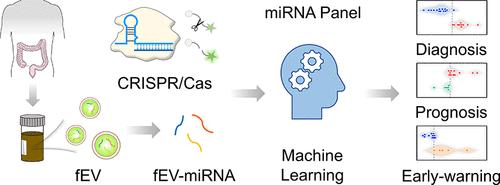
Machine Learning-Aided Identification of Fecal Extracellular Vesicle microRNA Signatures for Noninvasive Detection of Colorectal Cancer
Colorectal cancer (CRC) remains a formidable threat to human health, with considerable challenges persisting in its diagnosis, particularly during the early stages of the malignancy. In this study, we elucidated that fecal extracellular vesicle microRNA signatures (FEVOR) could serve as potent noninvasive CRC biomarkers. FEVOR was first revealed by miRNA sequencing, followed by the construction of a CRISPR/Cas13a-based detection platform to interrogate FEVOR expression across a diverse spectrum of clinical cohorts. Machine learning-driven models were subsequently developed within the realms of CRC diagnostics, prognostics, and early warning systems. In a cohort of 38 CRC patients, our diagnostic model achieved an outstanding accuracy of 97.4% (37/38), successfully identifying 37 of 38 CRC cases. This performance significantly outpaced the diagnostic efficacy of two clinically established biomarkers, CEA and CA19-9, which showed accuracies of mere 26.3% (10/38) and 7.9% (3/38), respectively. We also examined the expression levels of FEVOR in several CRC patients both before and after surgery, as well as in patients with colorectal adenomas (CA). Impressively, the results showed that FEVOR could serve as a robust prognostic indicator for CRC and a potential predictor for CA. This endeavor aimed to harness the predictive power of FEVOR for enhancing the precision and efficacy of CRC management paradigms. We envision that these findings will propel both foundational and preclinical research on CRC, as well as clinical studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


