通过 siRNA 处理降低 HBsAg 对自然和疫苗(BRII-179)诱导的 HBV 特异性体液和细胞免疫反应的影响
IF 25.7
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
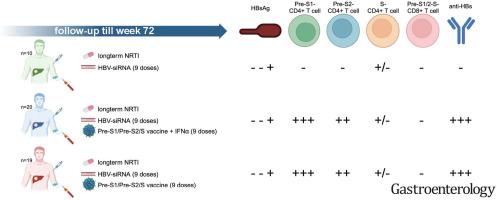
The impact of HBsAg reduction via siRNA treatment on natural and vaccine (BRII-179)-induced HBV-specific humoral and cellular immune responses
Background and aims
The impact of HBsAg reduction from small interfering RNA (siRNA) treatments on HBV-specific immunity of CHB participants has not been adequately analyzed in human. We conducted a phase 2a study treating CHB participants with 9 4-weekly doses of HBV-targeted siRNA elebsiran (BRII-835), either alone (n=10) or in combination with a VLP-based therapeutic vaccine (BRII-179) containing Pre-S1, Pre-S2, and S antigens, co-administered with (n=39) or without (n=41) IFNα.Methods
We analyzed longitudinally for 72 weeks virological, clinical, and immunological parameters including HBsAg, ALT, anti-HBs, the neutralizing activity of representative sera, and frequency and cytokine secretion ability of T cells specific for Pre-S1, Pre-S2, and S both directly ex vivo and after in vitro expansion.Results
Combination therapy of elebsiran and BRII-179 was well tolerated. While no sustained HBsAg seroclearance or notable difference in mean HBsAg reduction at the group level was observed, we detected marked heterogeneity in immunological responses among groups. HBsAg reduction mediated by siRNA alone was associated with minimal HBV-specific immune response recovery. In contrast, combination of elebsiran with BRII-179 induced a significant modification of immune responses demonstrated by anti-HBs antibody production and an expansion of IL-2-producing CD4+ T cells specific for Pre-S1/Pre-S2 antigens only. Importantly, anti-HBs antibodies persisted ≥100 IU/L in approximately 40% of the participants for at least 32 weeks after combinatory treatment. Moreover, the neutralizing ability of the anti-HBs positive sera was associated with HBsAg reduction.Conclusions
SiRNA-induced HBsAg reduction may contribute to the persistence and efficacy of the humoral arm of HBV-specific adaptive immunity in CHB participants receiving therapeutic vaccine BRII-179.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gastroenterology
医学-胃肠肝病学
CiteScore
45.60
自引率
2.40%
发文量
4366
审稿时长
26 days
期刊介绍:
Gastroenterology is the most prominent journal in the field of gastrointestinal disease. It is the flagship journal of the American Gastroenterological Association and delivers authoritative coverage of clinical, translational, and basic studies of all aspects of the digestive system, including the liver and pancreas, as well as nutrition.
Some regular features of Gastroenterology include original research studies by leading authorities, comprehensive reviews and perspectives on important topics in adult and pediatric gastroenterology and hepatology. The journal also includes features such as editorials, correspondence, and commentaries, as well as special sections like "Mentoring, Education and Training Corner," "Diversity, Equity and Inclusion in GI," "Gastro Digest," "Gastro Curbside Consult," and "Gastro Grand Rounds."
Gastroenterology also provides digital media materials such as videos and "GI Rapid Reel" animations. It is abstracted and indexed in various databases including Scopus, Biological Abstracts, Current Contents, Embase, Nutrition Abstracts, Chemical Abstracts, Current Awareness in Biological Sciences, PubMed/Medline, and the Science Citation Index.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


