多发性硬化症病变中神经元体细胞突变增多
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
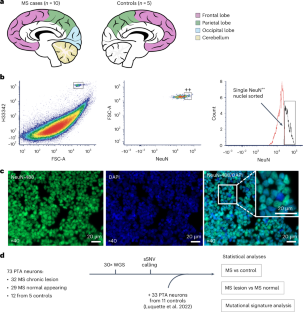
神经炎症是多发性硬化症(MS)神经退行性变和临床进展的基础,但有关这些疾病机制的过程的知识仍然不完整。本文研究了10例MS患者和16例对照患者死后脑组织106个神经元基因组中的体细胞单核苷酸变异(sSNVs),以确定体细胞突变是否参与其中。我们观察到,慢性MS病变神经元每年增加43.9个sSNVs,比正常MS和对照组织神经元的速度快2.5倍。与对照组相比,这一差异相当于70岁时病变神经元中ssnv增加了1291个。我们进行了突变特征分析,以研究神经元sSNV的机制,并确定了病变的一个特征,该特征对sSNV计数具有强烈的年龄相关贡献。这项研究表明,神经炎症在多发性硬化症大脑中具有诱变性,可能导致疾病进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Neuronal somatic mutations are increased in multiple sclerosis lesions
Neuroinflammation underpins neurodegeneration and clinical progression in multiple sclerosis (MS), but knowledge of processes linking these disease mechanisms remains incomplete. Here we investigated somatic single-nucleotide variants (sSNVs) in the genomes of 106 single neurons from post-mortem brain tissue of ten MS cases and 16 controls to determine whether somatic mutagenesis is involved. We observed an increase of 43.9 sSNVs per year in neurons from chronic MS lesions, a 2.5 times faster rate than in neurons from normal-appearing MS and control tissues. This difference was equivalent to 1,291 excess sSNVs in lesion neurons at 70 years of age compared to controls. We performed mutational signature analysis to investigate mechanisms underlying neuronal sSNVs and identified a signature characteristic of lesions with a strong, age-associated contribution to sSNV counts. This research suggests that neuroinflammation is mutagenic in the MS brain, potentially contributing to disease progression. The link between neuroinflammation and the progression of multiple sclerosis (MS) is unclear. Here, the authors show that in MS lesions, neuronal somatic mutations accumulate 2.5 times faster than in controls, equivalent to 1,291 excess mutations by age 70, suggesting that neuroinflammation can be mutagenic.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


