含银NH2-MIL-101(Cr)电极,通过假电容去离子高效去除碘化物
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
利用法拉第反应的电容去离子(CDI)技术由于其增强离子选择性的能力而获得了极大的兴趣。本研究采用浸渍银的NH2-MIL-101(Cr)作为电极的活性材料,实现高效除碘和电化学再生,从而实现持续的吸附-脱附循环。浸渍银的NH2-MIL-101(Cr)表现出优异的碘选择性,即使在负离子的摩尔比高达碘离子的103倍的竞争溶液中,其分布系数也超过104。本研究还发现,碘离子的最终吸附状态随外加电压的变化而变化,利用这一点,碘化物可以转化为三碘化物进行吸附,从而获得615 mg g−1的高吸附容量。此外,在吸附过程中证实了浸渍银的增强催化作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
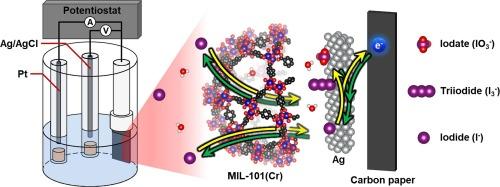
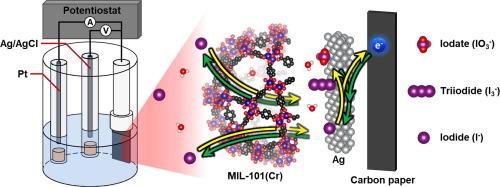
Silver-incorporated NH2-MIL-101(Cr) electrode for highly efficient iodide removal through pseudocapacitive deionization
Capacitive deionization (CDI) technology utilizing faradaic reactions has garnered substantial interest due to its ability for enhanced ion selectivity. In this study, silver-impregnated NH2-MIL-101(Cr) was employed as the active material for the electrode to achieve efficient iodide removal and electrochemical regeneration, thereby enabling sustainable adsorption–desorption cycling. The silver-impregnated NH2-MIL-101(Cr) exhibited exceptional iodide selectivity, with a distribution coefficient exceeding 104, even in solutions with competing anions at molar ratios as high as 103 times that of iodide. This study also discovered that the final adsorption state of iodine ions varies with the applied voltage, and by leveraging this, iodide can be converted triiodide for adsorption, resulting in a high adsorption capacity of 615 mg g−1. Additionally, the enhanced catalytic role of impregnated silver was confirmed during the adsorption process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


