氧空位诱导捕获效应增强过氧乙酸活化和选择性高价钴氧物种形成
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
高价钴氧(CoIV=O)由于其抗干扰性和选择性降解性而受到越来越多的研究;然而,克服Co三维轨道的高占位率以选择性地形成CoIV=O仍然是一个挑战。本文通过酸蚀策略在Co3O4 (OV-Co)表面设计氧空位(OVs),以增强过氧乙酸(PAA)的活化。OV-Co-8/PAA体系在60 min内完全降解磺胺甲恶唑(SMX), kobs值(0.07612 min−1)是原始Co3O4 (CK-Co)/PAA体系(0.01082 min−1)的7倍。甲基苯基亚砜(PMSO)作为分子探针,证实ov诱导形成CoIV=O,在OV-Co-8/PAA体系中主导降解过程。原位拉曼光谱和理论计算表明,OVs可以通过俘获效应诱导吸附在Co位点的PAA的O-O键断裂,导致Co位点的共配(CH3CO2-Co-OH)。这种共配促进了Co位点的电子通过Co dz2-O pz通道(CH3CO2-Co)转移,稳定了先前不利的Co - O反键轨道,促进了CoIV=O的形成。从热力学角度来看,OVs降低了CH3CO2释放所需的能量,并通过质子耦合电子转移(PCET)途径促进了H的转移。此外,OV-Co-8被装载到碳毡膜上用于水净化,实现了低钴浸出的SMX连续降解。本研究促进了对PAA活化过程中表面缺陷的分子水平机理的认识,指导了高效环境催化剂的合理设计。本文章由计算机程序翻译,如有差异,请以英文原文为准。
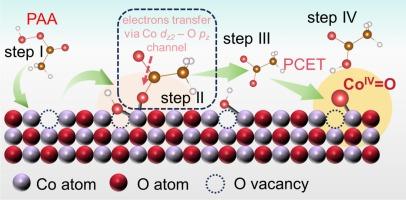
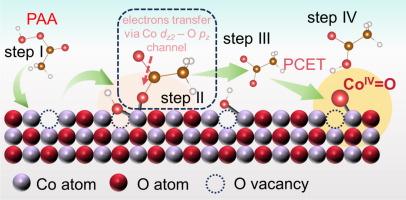
Oxygen vacancies induced capture effect for enhancing peracetic acid activation and selective high-valent cobalt-oxo species formation
High-valent cobalt-oxo species (CoIV=O) is being increasingly investigated due to its anti-interference properties and selective degradability; however, overcoming the high occupancy of the Co 3d-orbitals to selectively form CoIV=O remains a challenge. Herein, oxygen vacancies (OVs) were engineered on the surface of Co3O4 (OV-Co) via an acid etching strategy to enhance the activation of peracetic acid (PAA). The OV-Co-8/PAA system achieved complete degradation of sulfamethoxazole (SMX) within 60 min, with a kobs value (0.07612 min−1) 7 times higher than that of the pristine Co3O4 (CK-Co)/PAA system (0.01082 min−1). Methyl phenyl sulfoxide (PMSO), used as a molecular probe, confirmed the OV-induced formation of CoIV=O, which dominated the degradation process in the OV-Co-8/PAA system. Situ Raman spectroscopy and theory calculations indicated that OVs can induce the cleavage of the O–O bond in PAA adsorbed at Co sites through a capture effect, resulting in the co-coordination at Co sites (CH3CO2-Co-OH). Such co-coordination facilitated electron transfer from the Co sites via the Co dz2–O pz channel (CH3CO2-Co), stabilizing the previously unfavorable Co–O anti-bonding orbitals and promoting CoIV=O formation. From a thermodynamic perspectives, OVs reduce the energy required for CH3CO2 release and facilitate the transfer of H through a proton-coupled electron transfer (PCET) pathway. Additionally, OV-Co-8 was loaded onto a carbon-felt membrane for water purification, achieving continuous SMX degradation with low cobalt leaching. This study advances the understanding of the molecular-level mechanism of surface defects in PAA activation and guides the rational design of efficient environmental catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


