轴向氯诱导对称破缺铁单原子电化学合成氨催化剂
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
电化学硝酸还原反应(NO3-RR)是一种可持续的氨合成方法。具有对称平面四配体结构(M-N4)的单原子催化剂是NO3-RR的有利催化活性位点。然而,标准M-N4结构固有的极端对称性限制了反应动力学。本文在氮掺杂碳(Cl-Fe-NC)上引入了一种轴向氯配位的对称破缺铁单原子催化剂。Cl-Fe-NC在- 0.28 V对可逆氢电极(RHE)时的氨法拉第效率(FE)为99.4%,在- 0.68 V对可逆氢电极(RHE)时的产率为9396.7 μgNH3 h-1 cm-2,显著优于FE - nc(80%,相同电位下为4330.9 μgNH3 h-1 cm-2)。Operando同步辐射傅里叶变换红外光谱(SR-FTIR)证实了关键中间体的形成,如*NO, *NO- hx和σ(N-H)。密度泛函理论(DFT)计算将NO3-RR中间体的优化自由能归因于轴向氯设计,使势测定步骤势垒能降低了0.66 eV。轴向Cl原子的存在调节了单个Fe原子的对称性,增强了NO3-RR过程中硝酸盐离子的吸附和关键中间体的富集,抑制了析氢反应(HER)。这一发现为通过掺杂杂原子进行对称破缺来精确调制原子结构来促进电化学氨合成开辟了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
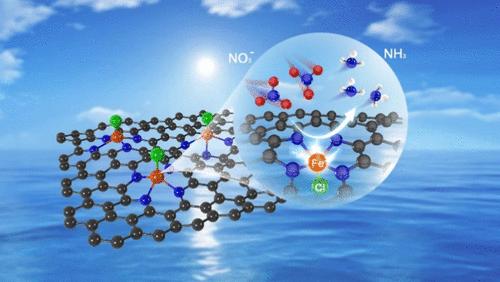
Axial Chlorine-Induced Symmetry-Breaking Iron Single-Atom Catalyst for Electrochemical Ammonia Synthesis
Electrochemical nitrate reduction reaction (NO3–RR) presents a sustainable method for ammonia synthesis. Single-atom catalysts possessing the symmetric planar four-ligand structure (M-N4) serve as advantageous catalytic active sites for NO3–RR. However, the inherent extreme symmetry of the standard M-N4 structure limits the reaction kinetics. Herein, we introduce a symmetry-breaking iron single-atom catalyst coordinated with axial chlorine on nitrogen-doped carbon (Cl-Fe-NC) for NO3–RR. Cl-Fe-NC exhibits a 99.4% ammonia Faradaic efficiency (FE) at −0.28 V vs reversible hydrogen electrode (RHE) with a 9396.7 μgNH3 h–1 cm–2 yield rate at −0.68 V vs RHE, remarkably surpassing that of Fe-NC (<80%, 4330.9 μgNH3 h–1 cm–2 at the same potential). Operando synchrotron radiation Fourier transform infrared (SR-FTIR) spectroscopy confirms that key intermediates, such as *NO, *NO-Hx, and σ(N–H), are formed. Density functional theory (DFT) calculations attribute the optimized free energy of NO3–RR intermediates to the axial chlorine design, reducing the potential determination step barrier energy by up to 0.66 eV. The presence of axial Cl atoms modulates the symmetry of the single Fe atom, enhancing the adsorption of nitrate ions and the enrichment of critical intermediates during NO3–RR while inhibiting the hydrogen evolution reaction (HER). This discovery opens avenues for boosting electrochemical ammonia synthesis through the precise modulation of atomic structures by doping heteroatoms for symmetry breaking.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


