hsd17b13介导的肝细胞自噬对肝缺血/再灌注损伤的生理调控
IF 26.8
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
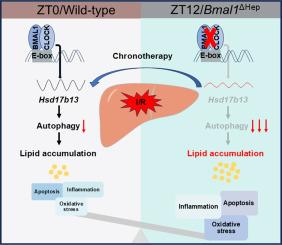
背景,研究表明昼夜节律在肝缺血/再灌注损伤(HIRI)中的作用,但其机制尚不清楚。Bmal1是在肝脏生理和疾病的昼夜节律控制中发挥重要作用的最重要基因,然而,其在HIRI中的作用尚未被研究。在这里,我们旨在探讨BMAL1对HIRI的潜在贡献。方法观察缺血再灌注(I/R)时间(ZT0 vs. ZT12)对Bmal1在肝细胞或髓细胞特异性缺失小鼠肝损伤的影响。采用RNA测序等多种分子生物学实验,探索其分子机制。此外,我们研究了HSD17B13(一种脂滴相关蛋白)在bmal1介导的HIRI昼夜节律控制中的作用,通过使用全局敲除、肝细胞特异性敲除或肝细胞特异性人源化HSD17B13过表达小鼠模型。结果我们发现,与ZT0相比,在ZT12开始I/R手术导致野生型小鼠肝损伤明显更严重。肝细胞中的Bmal1介导了这种时间差异,而髓细胞中没有。在机制上,BMAL1通过结合e -box样元件直接控制Hsd17b13的转录,从而调节HIRI的日振荡。肝细胞特异性敲低Hsd17b13抑制了HIRI的日变化,加重了ZT0 HIRI。此外,BMAL1/HSD17B13轴的缺失可能通过阻断自噬通量来抑制脂质降解,从而导致脂质过载并加剧HIRI。最后,我们证明了人源化HSD17B13的肝细胞特异性过表达可能在ZT0 HIRI期间提供保护,但在ZT12时加重损伤。结论我们的研究揭示了肝细胞BMAL1通过HSD17B13介导的自噬调节HIRI昼夜节律的关键作用,并为靶向BMAL1/HSD17B13轴预防和治疗HIRI提供了有希望的策略。影响和意义本研究揭示了BMAL1/HSD17B13轴在肝缺血再灌注损伤的昼夜节律控制中的关键作用,为肝缺血再灌注损伤的预防和治疗提供了新的见解。这些发现具有科学意义,因为它们增强了我们对肝脏缺血/再灌注损伤的昼夜节律调节的理解。此外,在临床上,本研究通过考虑治疗干预的时机,为优化肝缺血/再灌注损伤的治疗策略提供了机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Circadian Control of Hepatic Ischemia/Reperfusion Injury via HSD17B13-Mediated Autophagy in Hepatocytes
Background & Aims
Studies have illustrated the role of circadian rhythm in hepatic ischemia/reperfusion injury (HIRI), but the mechanisms are poorly understood. Bmal1 is the most important gene that plays significant roles in the circadian control of liver physiology and disease, however, its role in HIRI has not been investigated. Here, we aimed to explore the potential contribution of BMAL1 to HIRI.Methods
The impact of ischemia/reperfusion (I/R) timing (ZT0 vs. ZT12) on liver damage were assessed in mice with Bmal1 specifically depleted in hepatocytes or myeloid cells. RNA sequencing and other multiple molecular biology experiments were employed to explore the molecular mechanisms. Additionally, we investigated the role of HSD17B13, a lipid droplet-associated protein, in BMAL1-mediated circadian control of HIRI by utilizing global knockout, hepatocyte-specific knockdown, or hepatocyte-specific humanized HSD17B13 overexpression mouse models.Results
We found that initiating I/R operations at ZT12 resulted in significantly more severe liver injury in wild-type mice compared to ZT0. Bmal1 in hepatocytes, but not in myeloid cells, mediated this temporal difference. Mechanistically, BMAL1 regulates the diurnal oscillation of HIRI by directly controlling Hsd17b13 transcription via binding to E-box-like elements. Hepatocyte-specific knockdown of Hsd17b13 blunted the diurnal variation of HIRI and exacerbated ZT0 HIRI. Furthermore, depletion of the BMAL1/HSD17B13 axis may inhibit lipid degradation by blocking autophagy flux, contributing to lipid overload and exacerbating HIRI. Finally, we demonstrated that hepatocyte-specific overexpression of humanized HSD17B13 may confer protection during ZT0 HIRI but aggravate damage at ZT12.Conclusions
Our study uncovers a pivotal role of hepatocyte BMAL1 in modulating circadian rhythms in HIRI via HSD17B13-mediated autophagy and offers a promising strategy for preventing and treating HIRI by targeting the BMAL1/HSD17B13 axis.Impact and implications
This study unveils a pivotal role of the BMAL1/HSD17B13 axis in the circadian control of hepatic ischemia/reperfusion injury, providing new insights into the prevention and treatment of hepatic ischemia/reperfusion injury. The findings have scientific implications as they enhance our understanding of the circadian regulation of hepatic ischemia/reperfusion injury. Furthermore, clinically, this research offers opportunities for optimizing treatment strategies in hepatic ischemia/reperfusion injury by considering the timing of therapeutic interventions.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


