一种新的电化学霍夫曼型重排使得α-氧异氰酸酯易于合成n-氨基甲酰乙酰胺†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
由于通常使用的溴化催化剂和胺之间的氧化电位差异很小,导致胺失活,因此在霍夫曼重排中利用胺作为亲核试剂仍然是电化学领域的一个长期挑战。在此,我们展示了一种前所未有的霍夫曼式重排,在电化学条件下,以TBAI作为唯一添加剂,可以方便地从现成的α-氧酰胺中获得具有挑战性的α-氧异氰酸酯。研究了多种伯胺和仲胺作为有效的偶联伙伴,以提供具有优异化学选择性和满意收率的增值n -氨基甲酰乙酰胺。此外,该方法容易产生n -氨基甲酸酯和醇亲核试剂。这种原子/电子经济,可扩展的方法具有操作简单,衬底范围广,功能基团耐受性好等特点。这一策略的潜在效用已通过其在药物和杀虫剂的简明合成中的适用性得到阐明。这种电化学方法的成功源于其独特的机制,即通过TBAI催化剂和亲核试剂的共同作用,将α-氧酰胺转化为α-氧异氰酸酯,而不是传统霍夫曼重排中通常采用的直接酰胺活化。此外,TBAI和阴极还原的选择对相变至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
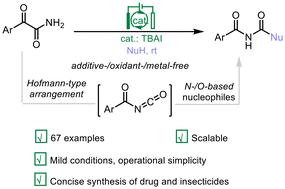
A novel electrochemical Hofmann-type rearrangement enables facile access to α-oxoisocyanates for the synthesis of N-carbamoylacetamides†
The utilization of amines as nucleophiles in Hofmann rearrangement remains a persistent challenge in the field of electrochemistry due to the small difference in oxidation potential between the commonly employed bromide catalysts and amines that would lead to amine deactivation. Herein, we demonstrate an unprecedented Hofmann-type rearrangement that allows convenient access to the challenging α-oxoisocyanates from readily available α-oxoamides with TBAI as the only additive under electrochemical conditions. A variety of primary and secondary amines were examined as effective coupling partners to afford value-added N-carbamoylacetamides with exceptional chemoselectivity and satisfactory yields. Besides, the protocol readily yields N-acylcarbamates with alcohol nucleophiles. This atom-/electron-economical, scalable method features operational simplicity, broad substrate scope, and excellent functional group tolerance. The potential utility of this strategy has been elucidated by its applicability in the concise synthesis of drugs and insecticides. The success of this electrochemical method stems from its unique mechanism to convert α-oxoamides into α-oxoisocyanates through the concerted efforts of TBAI catalysts and nucleophiles, rather than the direct amide activation commonly employed in traditional Hofmann rearrangement. Furthermore, the choice of TBAI and the cathodic reduction is crucial for the transformation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


