钼(VI)氧化物纳米线脱氧过程中直接和间接晶体学途径的共存
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
氧化钼(MoO3)基材料因其在催化和光电子学方面的重要应用而受到广泛关注。然而,在工作条件下,将氧空位引入MoO3材料不可避免地会导致其性能和性能的剧烈变化。因此,了解MoO3在受控环境下的脱氧过程势在必行。在此,我们利用先进的原位透射电子显微镜技术揭示了正交MoO3的脱氧机制。我们发现,在加热诱导脱氧过程中,MoO3材料可以遵循两种可能的晶体学途径。第一种是从正交MoO3到单斜MoO2的直接拓扑化学转变,而另一种是涉及单斜MoO3中间相的间接转变。这两种途径的共存可以用元素扩散和相变之间的动力学竞争来解释。我们的发现为钼氧化物的脱氧过程提供了基本的见解,并为其结构设计提供了潜在的指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
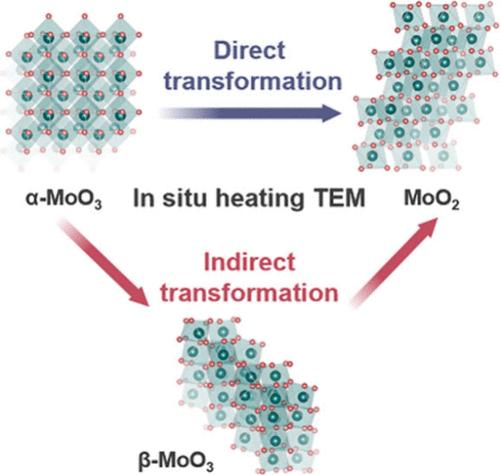
Coexistence of Direct and Indirect Crystallographic Pathways in Deoxidation of Molybdenum(VI) Oxide Nanowires
Molybdenum oxide (MoO3)-based materials have attracted considerable attention due to their significant applications in catalysis and optoelectronics. However, the introduction of oxygen vacancies into MoO3 materials under working conditions inevitably results in drastic changes in their properties and performance. Therefore, it is imperative to comprehend the deoxidation process of MoO3 in controlled environments. Herein, we reveal the deoxidation mechanisms of orthorhombic MoO3 using an advanced in situ transmission electron microscopy technique. We found that during heating-induced deoxidation, MoO3 material can follow two possible crystallographic pathways. The first is a direct topochemical transformation from orthorhombic MoO3 into monoclinic MoO2, whereas the other is an indirect transition involving a monoclinic MoO3 intermediate phase. The coexistence of these two pathways can be explained by the kinetic competition between elemental diffusion and phase transformation. Our findings provide fundamental insight into the deoxidation process of molybdenum oxides and offer potential guidance for their structural design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


