凝析油中PRC1压实染色质的染色质网格模型
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
多梳抑制复合体1 (Polycomb repression complex 1, PRC1)结合的染色质结构及其对转录抑制的作用尚不清楚。在这里,prc1 -染色质凝聚物的冷冻电子断层扫描(cro - et)显示,在一个稳定、开放的染色质网中,随机定向的邻近核小体可能会排除大的蛋白质复合物来抑制转录。本文章由计算机程序翻译,如有差异,请以英文原文为准。

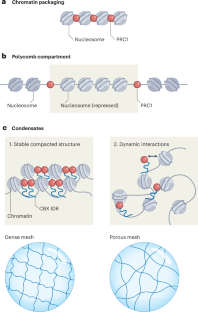
A chromatin mesh model for compaction of chromatin by PRC1 in condensates
The structure of chromatin bound by Polycomb repressive complex 1 (PRC1) and how it contributes to transcription repression are unknown. Here, cryo-electron tomography (cryo-ET) of PRC1–chromatin condensates reveal randomly oriented neighboring nucleosomes in a stable, open chromatin mesh that might exclude large protein complexes to repress transcription.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


