adsl生成的富马酸盐结合并抑制STING促进肿瘤免疫逃逸
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
高侵袭性肿瘤已经进化到抑制cGAS-STING途径的免疫逃避,这种劫持的机制尚不清楚。在这里,我们证明了缺氧在正常乳腺上皮细胞中诱导强大的STING激活,但在乳腺癌细胞中没有。腺苷琥珀酸裂解酶(adenylosuccinate lyase, ADSL)是新生嘌呤合成的关键代谢酶,在乳腺癌组织中高表达,并在T350位点被缺氧激活的IKKβ磷酸化。磷酸化的ADSL在内质网与STING相互作用,ADSL产生的富马酸盐与STING结合,导致cGAMP与STING结合、STING激活以及随后的irf3依赖性细胞因子基因表达受到抑制。破坏ADSL-STING结合可促进STING激活并抑制肿瘤生长。值得注意的是,ADSL内质网易位阻断肽和抗pd -1抗体联合治疗可诱导肿瘤生长的累加性抑制作用,并显著增强免疫反应。值得注意的是,ADSL T350磷酸化水平与STING激活水平呈负相关,并预示乳腺癌患者预后不良。这些发现强调了代谢物富马酸在抑制STING激活中的关键作用,并揭示了通过靶向adsl -月光功能介导的STING抑制来改善免疫检查点治疗的新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。

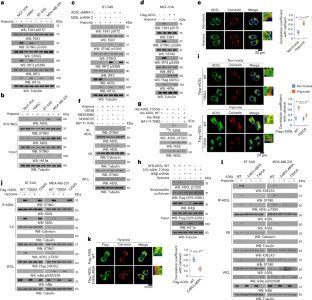
ADSL-generated fumarate binds and inhibits STING to promote tumour immune evasion
Highly aggressive tumours have evolved to restrain the cGAS–STING pathway for immune evasion, and the mechanisms underlying this hijacking remain unknown. Here we demonstrate that hypoxia induces robust STING activation in normal mammary epithelial cells but not in breast cancer cells. Mechanistically, adenylosuccinate lyase (ADSL), a key metabolic enzyme in de novo purine synthesis, is highly expressed in breast cancer tissues and is phosphorylated at T350 by hypoxia-activated IKKβ. Phosphorylated ADSL interacts with STING at the endoplasmic reticulum, where ADSL-produced fumarate binds to STING, leading to the inhibition of cGAMP binding to STING, STING activation and subsequent IRF3-dependent cytokine gene expression. Disrupting the ADSL–STING association promotes STING activation and blunts tumour growth. Notably, a combination treatment with ADSL endoplasmic reticulum translocation-blocking peptide and anti-PD-1 antibody induces an additive inhibitory effect on tumour growth accompanying a substantially increased immune response. Notably, ADSL T350 phosphorylation levels are inversely correlated with levels of STING activation and predicate poor prognosis in patients with breast cancer. These findings highlight a pivotal role of the metabolite fumarate in inhibiting STING activation and uncover new strategies to improve immune-checkpoint therapy by targeting ADSL-moonlighting function-mediated STING inhibition. Duan, Hu, Han, Lei, Wang et al. report that ADSL-mediated production of fumarate suppresses STING activation and impairs T cell and NK cell infiltration, thereby facilitating immune evasion in breast cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


