完整心脏的心脏兴奋性和电偶联的光遗传学量化解释心律失常的起始
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
Science Advances
Pub Date : 2025-02-28
引用次数: 0
摘要
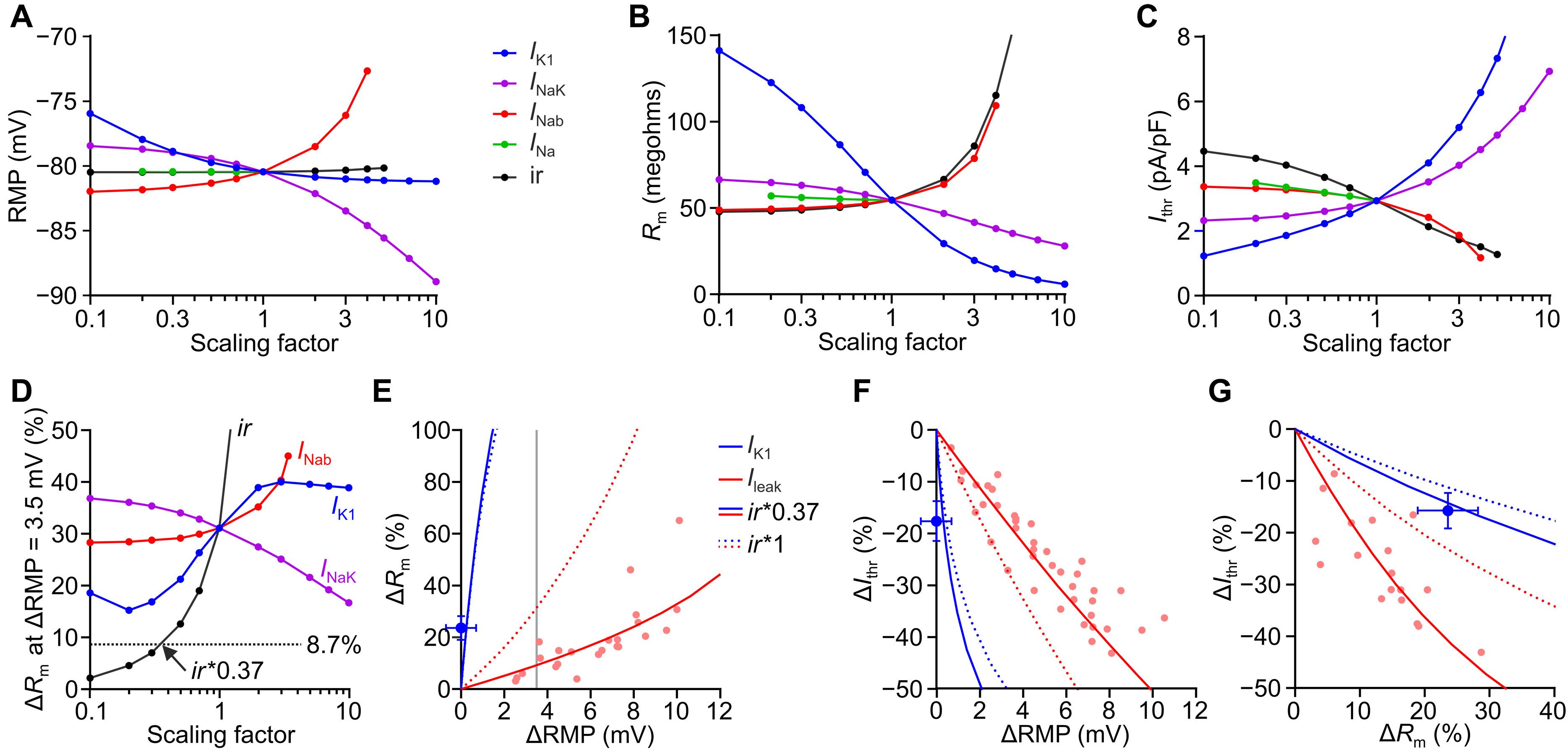
心脏兴奋性增加和电耦合减少可促进心律失常,可通过输入电阻(Rm)、起搏阈值(Ithr)和心脏空间常数(λ)来量化。然而,在心脏中进行测量是不可行的,因为所需的均匀电流注射不能通过电刺激进行。我们通过在小鼠心脏的不同动作电位阶段向所有被照射的心肌细胞注射光遗传电流,克服了这一问题。精确触发和图案照明可以测量Rm和λ,两者在舒张时都是最小的。药理学和去极化诱导的内整流K+电流(IK1)、间隙连接阻滞和心肌梗死的减少降低了Ithr,表明高IK1密度和完整的心肌细胞偶联对于预防心律失常起始的重要性。结合光遗传电流注入和计算机模拟,根据其对Rm和Ithr的影响对心律失常的促进和抗心律失常机制进行分类,并允许在完整心脏中量化IK1向内整流,确定减少IK1整流为抗心律失常概念。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Optogenetic quantification of cardiac excitability and electrical coupling in intact hearts to explain cardiac arrhythmia initiation
Increased cardiac excitability and reduced electrical coupling promote cardiac arrhythmia and can be quantified by input resistance (Rm), pacing threshold (Ithr), and cardiac space constant (λ). However, their measurement in the heart was not feasible because the required homogenous current injection cannot be performed with electrical stimulation. We overcame this problem by optogenetic current injection into all illuminated cardiomyocytes of mouse hearts in different action potential phases. Precisely triggered and patterned illumination enabled measuring Rm and λ, which both were smallest at diastole. Pharmacological and depolarization-induced reduction of inwardly rectifying K+ currents (IK1), gap junction block, and cardiac infarction reduced Ithr, showing the importance of high IK1 density and intact cardiomyocyte coupling for preventing arrhythmia initiation. Combining optogenetic current injection and computer simulations was used to classify pro- and anti-arrhythmic mechanisms based on their effects on Rm and Ithr and allowed to quantify IK1 inward rectification in the intact heart, identifying reduced IK1 rectification as anti-arrhythmic concept.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


