绘制钠硫电池中的多硫化物图谱
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
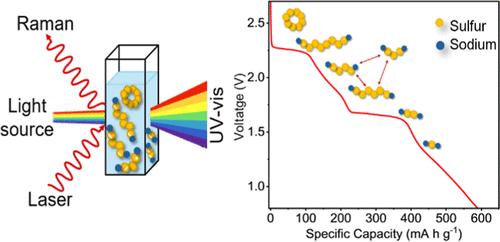
Mapping Polysulfides in Sodium–Sulfur Batteries
Sodium–sulfur (Na–S) batteries provide lithium-free alternatives to lithium–sulfur (Li–S) batteries. Na–S chemistry has been less studied. Thus, the types of polysulfides (PS) and their evolution during charge–discharge of Na–S batteries are not as well understood as those in the Li–S system. We, therefore, study the formation of different PS in tetraethylene glycol dimethyl ether-based electrolyte during battery operation using in situ Raman and ex situ ultraviolet–visible (UV–vis) spectroscopies. We start by making reference solutions with different ratios of sodium sulfide (Na2S) to sulfur, ranging from pure Na2S to Na2S:7S, with the sulfur ratio increasing by one integer per solution. We then correlate the UV–vis and Raman peaks to PS species. Our galvanostatic charge–discharge (GCD) and cyclic voltammetry measurements show a total of ten features. Using ex situ UV–vis on aliquots and in situ Raman spectra from PS solutions at GCD voltage plateaus, we map out sodium polysulfide (NaPS) species at key stages of the charge–discharge cycle. We identify Na2S8, Na2S4, and Na2S2 as intermediates and Na2S as the final product. We find that intermediate Na2S6 forms from disproportionation of Na2S8 and Na2S4. We also observe that intermediate PS can also dissociate into S3•– radical species, which contributes to loss of active material. Our results provide detailed insights into Na–S chemistry that will be helpful for the development of high performance and stable batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


