开发对氧气不敏感的 Nrf2 报告器揭示生理缺氧状态下的氧化还原调节机制
IF 8.2
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
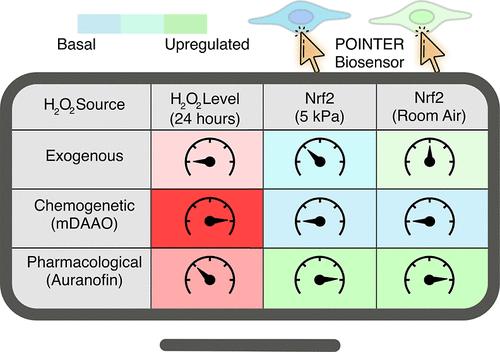
Development of an Oxygen-Insensitive Nrf2 Reporter Reveals Redox Regulation under Physiological Normoxia
Reactive oxygen species, particularly hydrogen peroxide (H2O2), play crucial roles in cellular signaling, with Nrf2 serving as a key transcription factor in maintaining redox homeostasis. However, the precise influence of H2O2 on Nrf2 activity under physiological normoxia remains unclear due to the limitations of oxygen-sensitive imaging methods. To address this, we developed and validated an oxygen-insensitive Nrf2 reporter named pericellular oxygen-insensitive Nrf2 transcriptional performance reporter (POINTER). We employed this reporter in human cerebral microvascular endothelial cells (hCMEC/D3). Using POINTER, we investigated how varying intracellular H2O2 concentrations affect Nrf2 regulation under normoxia (5 kPa O2) compared to hyperoxia (ambient air, 21 kPa O2). We manipulated intracellular H2O2 levels through exogenous application, chemogenetic production using a modified amino acid oxidase, and pharmacological induction with Auranofin. Our findings reveal that Nrf2 transcriptional activity is significantly lower under normoxia than under hyperoxia, supporting previous literature and expectations. Using POINTER, we found that both antioxidant pathway inhibition and sustained H2O2 elevation are essential for modulating Nrf2 activity. These findings provide new insights into the regulation of Nrf2 by H2O2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sensors
Chemical Engineering-Bioengineering
CiteScore
14.50
自引率
3.40%
发文量
372
期刊介绍:
ACS Sensors is a peer-reviewed research journal that focuses on the dissemination of new and original knowledge in the field of sensor science, particularly those that selectively sense chemical or biological species or processes. The journal covers a broad range of topics, including but not limited to biosensors, chemical sensors, gas sensors, intracellular sensors, single molecule sensors, cell chips, and microfluidic devices. It aims to publish articles that address conceptual advances in sensing technology applicable to various types of analytes or application papers that report on the use of existing sensing concepts in new ways or for new analytes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


