IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
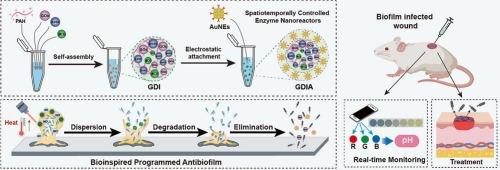
摘要 生物膜受其自身产生的致密基质的保护,因其抗生素耐药性而构成重大临床挑战,导致持续感染和伤口愈合延迟,尤其是在糖尿病患者中。针对生物膜的生命周期,我们开发了一种双层纳米反应器,用于快速、全面的抗生物膜治疗。内层与聚(烯丙胺盐酸盐)(PAH)/磷酸盐、二聚体吲哚菁绿(dICG)和溴百里酚蓝(BTB)交联,保护葡萄糖氧化酶(GOx)和β-葡聚糖酶(β-DEX)免受不利环境的影响。外层涂有细菌靶向金纳米酶(AuNEs)。受生物膜感染的糖尿病伤口的愈合在光照和 pH 值变化的激活下经历了三个时空阶段。最初,dICG 的光热效应会引发一氧化氮(NO)介导的生物膜分散,并通过 GOx/AuNEs 级联反应降低伤口的 pH 值。由此产生的酸性环境会诱导纳米反应器解体,释放出β-DEX,从而降解生物膜基质,促进更深的渗透。最后,AuNEs 能特异性识别并消灭浮游细菌,进一步破坏生物膜,并通过产生活性氧(ROS)和毒性更强的活性氮(RNS)加速伤口愈合。伤口状态可通过 BTB 的比色 pH 值分析进行实时监测,以获得治疗进展的视觉反馈。这种多功能设计为动态伤口管理提供了一种程序化的抗生物膜策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Bioinspired programmed antibiofilm strategies for accelerated wound healing via spatiotemporally controlled enzyme nanoreactors
Abstract
Biofilms, protected by their dense, self-produced matrix, pose a significant clinical challenge due to their antibiotic resistance, leading to persistent infections and delayed wound healing, particularly in diabetic patients. Tailored to the biofilm life cycle, a double-layered nanoreactor was developed for rapid and complete antibiofilm therapy. The inner layer, cross-linked with poly(allylamine hydrochloride) (PAH)/phosphate, dimeric indocyanine green (dICG), and bromothymol blue (BTB), shields glucose oxidase (GOx) and β-glucanase (β-DEX) from unfavorable environment. The outer layer is coated with bacteria-targeted gold nanozymes (AuNEs). The healing of biofilm-infected diabetic wounds progresses three spatiotemporal stages activated by light irradiation and pH changes. Initially, the photothermal effect of dICG triggers nitric oxide (NO)-mediated biofilm dispersion and lowers the wound pH via a GOx/AuNEs cascade reaction. The resulting acidic environment then induces nanoreactor disassembly, releasing β-DEX to degrade the biofilm matrix and facilitate deeper penetration. Finally, AuNEs specifically recognize and eliminate planktonic bacteria, further disrupting the biofilms and accelerating wound healing by generating reactive oxygen species (ROS) and more toxic reactive nitrogen species (RNS). The wound status can be monitored in real-time using BTB's colorimetric pH analysis for visual feedback on treatment progress. This multifunctional design offers a programmed antibiofilm strategy for dynamic wound management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


