IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
胰腺导管腺癌(PDAC)占胰腺癌的 90%,由于肿瘤微环境(TME)具有高度免疫抑制作用,因此对免疫疗法的反应有限。细胞因子编码的 mRNA 疗法在将 "冷 "肿瘤转化为 "热 "肿瘤方面大有可为,但它通常通过瘤内注射给药,仅适用于浅表肿瘤,这限制了其在 PDAC 中的应用。在这项研究中,我们引入了一种脂质纳米粒子(LNP)递送系统,它能通过腹腔注射靶向胰腺组织。该系统不仅能有效地将 mRNA 运送到胰腺组织,还能选择性地靶向 PDAC 中的免疫细胞。一次腹腔注射封装有白细胞介素-12(IL-12)mRNA的LNP,就能激活PDAC中的髓细胞和淋巴细胞,对免疫抑制性TME进行重编程。值得注意的是,在某些病例中,包裹有IL-12 mRNA的LNP(LNP/mIL-12)静脉注射可诱导根除正位PDAC。我们的工作代表了第一种相对非侵入性的递送IL-12 mRNA的方法,用于靶向治疗正位PDAC,为PDAC免疫疗法提供了一种新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
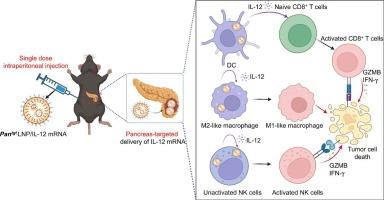
Pancreas-targeted lipid nanoparticles for relatively non-invasive interleukin-12 mRNA therapy in orthotopic pancreatic ductal adenocarcinoma
Pancreatic ductal adenocarcinoma (PDAC) represents 90 % of pancreatic cancers and shows limited response to immune therapy owing to the highly immunosuppressive tumor microenvironment (TME). Cytokine-encoded mRNA therapy demonstrates great promise in converting “cold” tumors into “hot” ones, while it is typically administered through intratumoral injection and applicable only to superficial tumors, which limites their application in PDAC. In this study, we introduce a lipid nanoparticle (LNP) delivery system capable of targeting pancreatic tissue via intraperitoneal (I·P.) injection. This system not only efficiently delivers mRNA to pancreatic tissues but also selectively targets immune cells in PDAC. A single I.P. injection of LNP encapsulating interleukin-12 (IL-12) mRNA activates both myeloid and lymphoid cells in PDAC, reprogramming the immunosuppressive TME. Remarkably, I.P. injection of LNP encapsulating IL-12 mRNA (LNP/mIL-12) induces eradication of orthotopic PDAC in some cases. Our work represents the first relatively non-invasive method to deliver IL-12 mRNA for targeted treatment of orthotopic PDAC, offering a novel approach for PDAC immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


