利用离子相互作用控制过渡金属催化选择性的设计方法
IF 51.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
两个带相反电荷的离子之间的吸引力可以成为选择性催化的强大设计工具。酶在广泛利用离子相互作用的同时,还利用了其他各种非共价相互作用;近年来,合成化学家们开始在含有活性过渡金属的催化剂设计中认真探索这些相互作用。孤立地看,单一离子相互作用的方向性很低,但在许多成功的体系中,它们与其他相互作用同时存在,可在决定选择性的过渡态上提供高度的组织性。即使在只有单一关键相互作用的情况下,低方向性也并非总是有害的,甚至可能是有利的,因为它可以赋予单一催化剂通用性。本综述探讨了利用离子相互作用控制过渡金属催化选择性的设计方法。它分为两部分:在第一部分中,离子相互作用发生在金属复合物的外球,使用的配体带电或与阴离子结合;在第二部分中,金属带有形式电荷,离子相互作用与相关的反离子有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
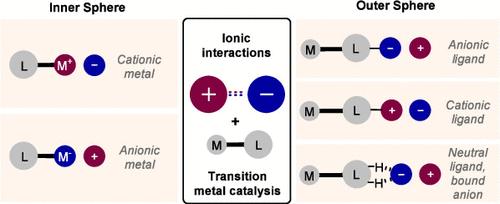
Design Approaches That Utilize Ionic Interactions to Control Selectivity in Transition Metal Catalysis
The attractive force between two oppositely charged ions can constitute a powerful design tool in selective catalysis. Enzymes make extensive use of ionic interactions alongside a variety of other noncovalent interactions; recent years have seen synthetic chemists begin to seriously explore these interactions in catalyst designs that also incorporate a reactive transition metal. In isolation, a single ionic interaction exhibits low directionality, but in many successful systems they exist alongside additional interactions which can provide a high degree of organization at the selectivity-determining transition state. Even in situations with a single key interaction, low directionality is not always detrimental, and can even be advantageous, conferring generality to a single catalyst. This Review explores design approaches that utilize ionic interactions to control selectivity in transition metal catalysis. It is divided into two halves: in the first, the ionic interaction occurs in the outer sphere of the metal complex, using a ligand which is charged or bound to an anion; in the second, the metal bears a formal charge, and the ionic interaction is with an associated counterion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Reviews
化学-化学综合
CiteScore
106.00
自引率
1.10%
发文量
278
审稿时长
4.3 months
期刊介绍:
Chemical Reviews is a highly regarded and highest-ranked journal covering the general topic of chemistry. Its mission is to provide comprehensive, authoritative, critical, and readable reviews of important recent research in organic, inorganic, physical, analytical, theoretical, and biological chemistry.
Since 1985, Chemical Reviews has also published periodic thematic issues that focus on a single theme or direction of emerging research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


