基于结构的新型LSD1/EGFRL858R/T790M双抑制剂治疗EGFR突变的NSCLC癌症
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
表观遗传改变,如LSD1失调,有助于EGFR突变的nsclc获得性耐药,并降低当前治疗方法的有效性。为了解决这些挑战,我们在此报道了基于结构的新型LSD1/EGFR双抑制剂的设计,其中ZJY-54代表了入围的先导化合物,具有高效,选择性和独特的双重作用模式(即与EGFR不可逆结合,但与LSD1可逆结合)。ZJY-54有效抑制亲本和tki耐药NSCLC细胞的生长。在H1975细胞中,ZJY-54诱导H3K4me2和H3K9me2的积累,并抑制EGFR信号的磷酸化。ZJY-54在H1975异种移植瘤模型中表现出良好的PK谱,有效抑制肿瘤生长。ZJY-54代表了同类最佳的LSD1/EGFR双抑制剂,值得进一步临床前开发用于治疗非小细胞肺癌。这些发现强调了LSD1/EGFR双抑制剂在EGFR和LSD1失调的耐药癌症中的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
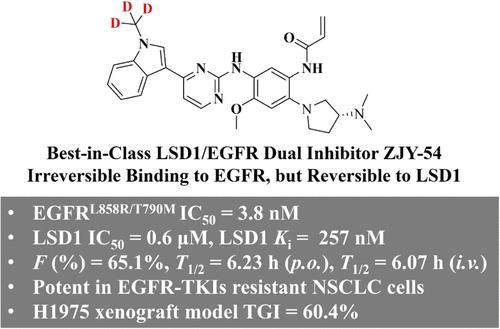
Structure-Based Design of New LSD1/EGFRL858R/T790M Dual Inhibitors for Treating EGFR Mutant NSCLC Cancers
Epigenetic changes, such as LSD1 dysregulation, contribute to acquired resistance in EGFR mutant NSCLCs and reduce the effectiveness of current therapeutics. To address the challenges, we herein reported the structure-based design of new LSD1/EGFR dual inhibitors, of which ZJY-54 represents the shortlisted lead compound with high potency, selectivity, and unique dual modes of action (namely irreversibly binding to EGFR but reversibly binding to LSD1). ZJY-54 effectively inhibited growth in both parent- and TKI-resistant NSCLC cells. In H1975 cells, ZJY-54 induced accumulation of H3K4me2 and H3K9me2, as well as inhibited phosphorylation of EGFR signaling. ZJY-54 showed favorable PK profiles and effectively inhibited tumor growth in the H1975 xenograft model. ZJY-54 represents the best-in-class LSD1/EGFR dual inhibitor and warrants further preclinical development for treating NSCLCs. These findings highlight the therapeutic potential of LSD1/EGFR dual inhibitors in drug-resistant cancers where EGFR and LSD1 were dysregulated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


