非齿状配体二乙二醇-双(3-氨基丙基醚)-N,N,N ',N ' -四乙酸DEGTA的合成及其与三价镧系元素和锕系元素的络合行为
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
采用两步法合成了聚氨基聚羧酸酯家族的新型无齿配体DEGTA(二乙二醇-双(3-氨基丙基醚)-N,N,N′,N′-四乙酸)。通过核磁共振(NMR)光谱测定了配体的ph依赖性行为(结构和pKa值)。以Eu(III)和Cm(III)为代表,采用时间分辨激光诱导荧光光谱(TRLFS)研究了该配体对三价镧系元素和锕系元素的络合能力。对于Eu(III),在不同的pH值下观察到两种物质,并通过浓度依赖性和pd依赖性核磁共振滴定系列得到证实,即[EuH2(DEGTA)]+和[Eu(DEGTA)]−。后者是九坐标的,分别与Cm(III)和Sm(III)形成同构配合物,分别由TRLFS和2D NMR实验推断。由于DEGTA可以看作是EDTA和EGTA的连续衍生物,具有细长的主链,因此使用密度泛函理论(DFT)计算了它们的Eu(III)配合物的结构,并通过傅里叶变换红外(FT-IR)光谱证明了相同的氨基乙酸结合基序。通过比较构效关系(密度和链长与配位几何和配合物稳定性),可以得出DEGTA的配位行为的结论,并讨论了配合物系列中配位性质的一些可推广的趋势。展望未来,这些知识将有助于进一步制定净化、退役和装饰策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
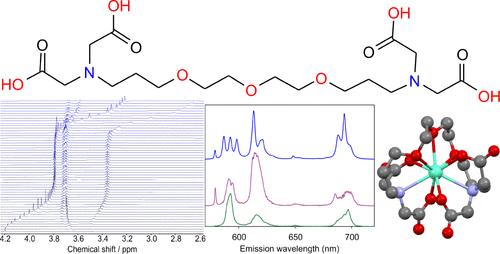
Synthesis of Nonadentate Ligand Diethylene Glycol-Bis(3-Aminopropyl Ether)-N,N,N′,N′-Tetraacetic Acid DEGTA and Its Complexation Behavior toward Trivalent Lanthanides and Actinides
A new nonadentate ligand, DEGTA (diethylene glycol-bis(3-aminopropyl ether)-N,N,N′,N′-tetraacetic acid), from the polyaminopolycarboxylate family, was synthesized in a two-step reaction. The ligand’s pH-dependent behavior (structure and pKa values) was determined by nuclear magnetic resonance (NMR) spectroscopy. The complexation ability of the ligand toward trivalent lanthanides and actinides was studied by time-resolved laser-induced fluorescence spectroscopy (TRLFS) using Eu(III) and Cm(III) as representatives. For Eu(III), two species occurring at different pH values were observed and corroborated by concentration- and pD-dependent NMR-titration series, viz. [EuH2(DEGTA)]+ and [Eu(DEGTA)]−. The latter is shown to be nine-coordinate, forming isostructural complexes with Cm(III) and Sm(III) as inferred from TRLFS and 2D NMR experiments, respectively. Since DEGTA can be seen as a consecutive derivative of EDTA and EGTA with an elongated backbone, the structures of their Eu(III) complexes were calculated using density functional theory (DFT) and the same aminoacetate binding motif proven by Fourier-transform infrared (FT-IR) spectroscopy. Upon comparison of structure–property relationships (denticity and chain length vs coordination geometry and complex stability) one can draw conclusions on DEGTA’s complexation behavior in particular, and some generalizable trends in complexation properties within the complexone series are discussed. Looking further ahead, this knowledge will help in further developing decontamination, decommissioning, and decorporation strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


