异常力依赖的转变率揭示了酰基辅酶A结合蛋白折叠和展开动力学的双重途径
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
所有α蛋白通常折叠迅速,无法承受高强度。酰基辅酶A结合蛋白(ACBP)是一种四α-螺旋束蛋白,是研究所有α蛋白折叠动力学的模型蛋白。在以往的生物化学和单分子力谱实验中,对于折叠途径和过渡态的构象存在争议。在本文中,我们使用磁镊子研究了ACBP在4-10 pN力范围内的折叠和展开动力学,揭示了异常的力依赖转变率。当外力低于6 pN时,ACBP的展开速率基本保持不变,当外力超过该阈值时,ACBP的展开速率急剧增加,而其折叠速率的对数几乎是外力的线性函数。结合分子动力学模拟的详细分析表明,ACBP有两种转变途径:一种是在零或低力下占优势,另一种是在高力下占优势。我们的研究结果提供了强有力的证据,证明拉伸力不仅可以调节折叠和展开速率,还可以改变过渡路径,从而导致复杂的力响应行为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
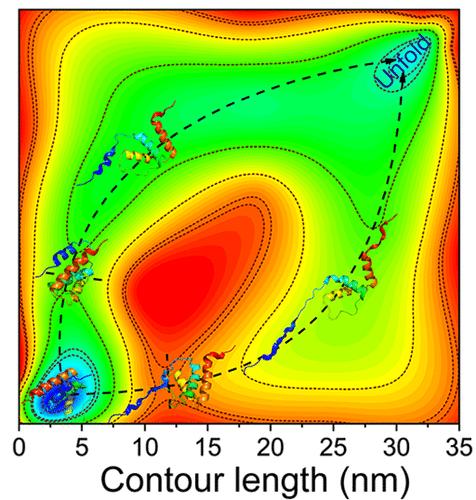
Anomalous Force-Dependent Transition Rates Unveil Dual Pathways in Folding and Unfolding Dynamics of Acyl-coenzyme A Binding Protein
All-α proteins typically fold rapidly and are unable to withstand high forces. Acyl-coenzyme A binding protein (ACBP), a four-α-helix bundle protein, serves as a model protein for studying the folding dynamics of all-α proteins. In previous biochemistry and single molecule force spectroscopy experiments, a controversy exists for the folding pathway and the conformation of the transition state. In this article, we investigate the folding and unfolding dynamics of ACBP in a force range of 4–10 pN using magnetic tweezers, revealing anomalous force-dependent transition rates. The unfolding rate of ACBP remains nearly constant when force is below 6 pN, and it increases sharply when the force exceeds this threshold, while the logarithm of its folding rate is almost a linear function of force. Detailed analysis combined with molecular dynamics simulations indicates that ACBP has two transition pathways: one dominating at zero or low force and the other dominating at high force. Our results provide strong evidence that stretching force not only modulates the folding and unfolding rates but also switches the transition pathways, leading to complex force response behaviors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


