脾TNF-α信号增强了抗病毒NK细胞的先天向适应性转变
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
自然杀伤细胞(NK细胞)具有先天和适应性特征。在这里,我们研究了巨细胞病毒感染过程中NK细胞的跨组织活化,从而产生抗原特异性克隆扩增和长寿命记忆反应。感染后NK细胞的纵向跟踪和单细胞RNA测序显示脾脏活化增强,以及CD69lo前体细胞的早期形成,优先产生适应性NK细胞。脾NK细胞表现出肿瘤坏死因子α (TNF-α)信号传导增强和受体TNFR2表达增加,这与脾髓细胞TNF-α产生升高相一致。缺乏tnfr2的NK细胞表现出干扰素γ (IFN-γ)产生和扩增受损。TNFR2信号通路涉及两种不同的核因子κB (NF-κB)信号通路——先天效应NK细胞应答需要典型的NF-κB信号通路,而非典型的NF-κB信号通路则强制CD69lo适应性NK细胞前体分化。因此,在病毒感染期间,脾脏中的NK细胞启动促进了先天到适应性的转变,为在不同环境下产生适应性NK细胞免疫提供了途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
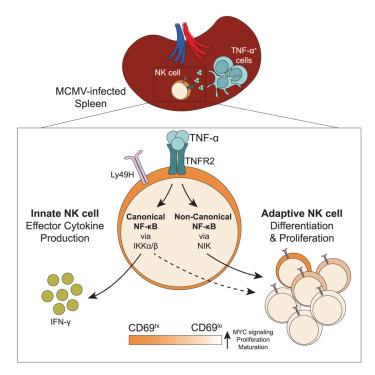
Splenic TNF-α signaling potentiates the innate-to-adaptive transition of antiviral NK cells
Natural killer (NK) cells possess both innate and adaptive features. Here, we investigated NK cell activation across tissues during cytomegalovirus infection, which generates antigen-specific clonal expansion and long-lived memory responses. Longitudinal tracking and single-cell RNA sequencing of NK cells following infection revealed enhanced activation in the spleen, as well as early formation of a CD69lo precursor population that preferentially gave rise to adaptive NK cells. Splenic NK cells demonstrated heightened tumor necrosis factor alpha (TNF-α) signaling and increased expression of the receptor TNFR2, which coincided with elevated TNF-α production by splenic myeloid cells. TNFR2-deficient NK cells exhibited impaired interferon gamma (IFN-γ) production and expansion. TNFR2 signaling engaged two distinct nuclear factor κB (NF-κB) signaling arms—innate effector NK cell responses required canonical NF-κB signaling, whereas non-canonical NF-κB signaling enforced differentiation of CD69lo adaptive NK cell precursors. Thus, NK cell priming in the spleen during viral infection promotes an innate-to-adaptive transition, providing insight into avenues for generating adaptive NK cell immunity across diverse settings.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


