利用与 IDO 抑制剂共轭的掺硫纳米碳点作为双模式乳腺癌免疫疗法
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。

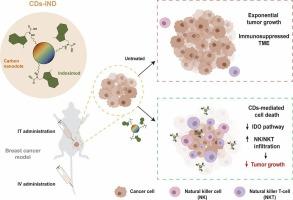
Harnessing sulfur-doped carbon nanodots conjugated with IDO inhibitors act as a dual-mode breast cancer immunotherapy
Fluorescent ultrasmall nanoparticles (d < 10 nm), such as carbon nanodots (CDs), are promising nanosystems for precision cancer therapy. Their optimal size allows them to diffuse within complex microenvironments, enabling drug delivery, imaging, and monitoring. Additionally, CDs can be engineered to hold inherent nanotoxicity toward cancer cells, overcoming multidrug resistance associated with conventional drugs. Nevertheless, cancer is a multifactorial disease where combinational strategies are most likely to tackle metastatic tumors and efficiently avoid recidivism. Therefore, developing multifunctional CDs that exhibit intrinsic nanotoxicity against cancer cells and drive effective antitumor immune responses is a promising approach to improving patients' response rates.
Here, we developed an innovative nanosystem by conjugating N-,S-doped CDs with indoximod (IND) through a simple and cost-effective method. Our CDs-IND not only retained the advantages of bare CDs, including photoluminescence for self-tracking but also significantly controlled breast cancer progression in vivo following CDs-IND intratumoral (IT) and intravenous (IV) administration. Tumor microenvironment (TME) immune profiling revealed that CDs-IND reduced IDO expression and recruited NK, NKT, and T cells. This study underscores the potential of combining the inherent pharmacological properties of CDs with indoximod-mediated immunotherapy, offering a promising strategy for precision breast cancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


