赖氨酸vitcyation是一种维生素c衍生的蛋白质修饰,可增强stat1介导的免疫反应
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
维生素C (vitC)对健康至关重要,在治疗癌症等疾病方面显示出希望,但其机制尚不清楚。在这里,我们报道了维生素c直接修饰赖氨酸残基形成“vitcyl-lysine”-一个被称为vitcyation的过程。在无细胞系统和活细胞中,卵细胞分裂以剂量、pH值和序列依赖的方式发生。从机制上说,vitC破坏转录-1 (STAT1)-赖氨酸-298 (K298)信号转导和激活因子,损害其与T细胞蛋白酪氨酸磷酸酶(TCPTP)的相互作用,阻止STAT1- y701去磷酸化。这导致肿瘤细胞中stat1介导的干扰素(IFN)信号传导增强,主要组织相容性复合体(MHC)/人白细胞抗原(HLA) I类表达增加,并激活体外和体内抗肿瘤免疫。vitcyation作为一种独特的翻译后修饰的发现,为vitC的细胞功能和治疗潜力提供了重要的见解,为理解其生物学效应和在疾病治疗中的应用开辟了途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
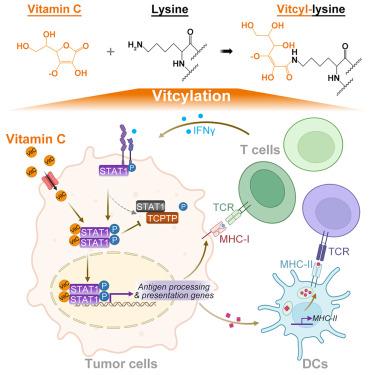
Lysine vitcylation is a vitamin C-derived protein modification that enhances STAT1-mediated immune response
Vitamin C (vitC) is essential for health and shows promise in treating diseases like cancer, yet its mechanisms remain elusive. Here, we report that vitC directly modifies lysine residues to form “vitcyl-lysine”—a process termed vitcylation. Vitcylation occurs in a dose-, pH-, and sequence-dependent manner in both cell-free systems and living cells. Mechanistically, vitC vitcylates signal transducer and activator of transcription-1 (STAT1)- lysine-298 (K298), impairing its interaction with T cell protein-tyrosine phosphatase (TCPTP) and preventing STAT1-Y701 dephosphorylation. This leads to enhanced STAT1-mediated interferon (IFN) signaling in tumor cells, increased major histocompatibility complex (MHC)/human leukocyte antigen (HLA) class I expression, and activation of anti-tumor immunity in vitro and in vivo. The discovery of vitcylation as a distinctive post-translational modification provides significant insights into vitC’s cellular function and therapeutic potential, opening avenues for understanding its biological effects and applications in disease treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


