早期流感病毒暴露影响50年后个体对流感疫苗接种的B细胞反应
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
预先存在的免疫会影响疫苗对流感的反应,但很难将生命早期的流感感染与几十年后的免疫反应直接联系起来。然而,H2N2在1968年停止在人群中传播,这为50年后直接评估早期接触H2N2对疫苗反应的影响创造了机会。在这里,我们用H2血凝素(HA) DNA质粒和/或铁蛋白纳米颗粒疫苗接种了1968年(H2暴露)之前或之后出生的个体。暴露于h2的个体产生了快速的B细胞召回反应,这种反应更有效,针对更保守的表位,并且在表型上不同于h2初始个体的从头反应。此外,DNA疫苗和蛋白质纳米颗粒疫苗分别改变了未接触h2的个体的反应,但没有改变暴露于h2的个体的反应。本研究建立并描述了生命早期形成的流感ha特异性记忆B细胞对几十年后疫苗反应的终身影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
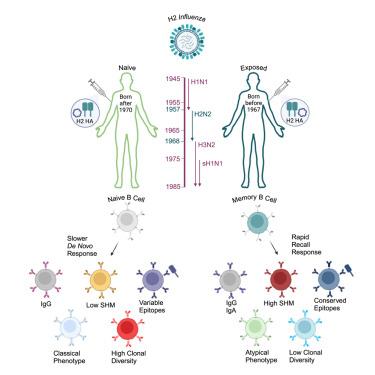
Early influenza virus exposure shapes the B cell response to influenza vaccination in individuals 50 years later
Pre-existing immunity impacts vaccine responses to influenza, but directly connecting influenza infections early in life with immune responses decades later is difficult. However, H2N2 stopped circulating in the human population in 1968, creating the opportunity to directly evaluate the impact of early H2N2 exposure on vaccine responses 50 years later. Here, we vaccinated individuals born before (H2 exposed) or after (H2 naive) 1968 with an H2 hemagglutinin (HA) DNA plasmid and/or a ferritin nanoparticle vaccine. H2-exposed individuals generated a rapid B cell recall response that was more potent, targeted more conserved epitopes, and differed phenotypically from the de novo response in H2-naive individuals. Furthermore, vaccinating with a DNA versus a protein nanoparticle vaccine altered the response in H2-naive but not H2-exposed individuals. This study establishes and describes the lifelong impact of influenza HA-specific memory B cells formed early in life on vaccine responses decades later.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


