过氧化氢处理的甘油源多孔碳与单质硫基硫磷共掺杂用于CO2捕获
IF 8
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
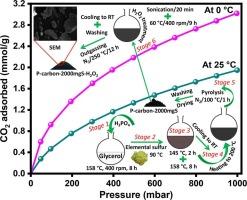
在这项工作中,开发了甘油和单质硫基多孔碳吸附剂,通过硫磷共掺杂和H2O2处理来捕获二氧化碳。所开发的吸附剂中,捕集CO2效果最好的吸附剂是P -碳- 2000mgs - h2o2,其比表面积为652 m2/g,总孔容为0.446 cm3/g,平均孔径为2.74 nm,微孔分布较窄,x射线光电子能谱(XPS)基硫含量为5.7 at。%,磷含量3.7 at。%,基于拉曼的PAHs平均尺寸为24.9 Å,缺陷密度为4.47 × 1011 cm−2,基于x射线衍射(XRD)的纳米晶体高度为11.15 Å,长度为23.35 Å。P -碳-2000mg - h2o2在25°C和1 bar条件下的CO2吸附量为1.95 mmol/g(0°C条件下为3.02 mmol/g),并且在25°C条件下对N2的CO2选择性也很好,在0.5 bar条件下为15.24,在1 bar条件下为12.03。除循环性能外,CO2吸附的等等热在22 ~ 23 kJ/mol之间,表明CO2与活性位点的相互作用主要是物理机制。这些发现表明,利用单质硫制备硫磷共掺杂的甘油衍生多孔碳,并进行H2O2处理,是一种有效的生产二氧化碳捕获吸附剂的方法,有利于甘油和单质硫基产品的大规模应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hydrogen peroxide-treated glycerol sourced porous carbon with elemental sulfur-based sulfur-phosphorus co-doping for CO2 capture
In this work, glycerol and elemental sulfur-based porous carbon adsorbents with sulfur‑phosphorus co-doping and subsequent H2O2 treatment were developed for CO2 capture. The best adsorbent for capturing CO2 among the developed adsorbents was P‑carbon-2000mgS-H2O2, which had surface area of 652 m2/g, a total pore volume of 0.446 cm3/g, an average pore size of 2.74 nm, narrow micropore distribution, X-ray photoelectron spectroscopy (XPS)-based sulfur content of 5.7 at.% and phosphorus content of 3.7 at.%, Raman-based average PAHs size of 24.9 Å and a defect density of 4.47 × 1011 cm−2, and X-ray diffraction (XRD)-based nano-crystallite height of 11.15 Å and length of 23.35 Å. The CO2 adsorption capacity of P‑carbon-2000mgS-H2O2 was 1.95 mmol/g at 25 °C and 1 bar (3.02 mmol/g at 0 °C), and it also demonstrated an impressive CO2 selectivity over N2 at 25 °C, with 15.24 at 0.5 bar and 12.03 at 1 bar. In addition to cyclic performance, the isosteric heat of CO2 adsorption, which was found to be between 22 and 23 kJ/mol, suggested that a physical mechanism predominated the CO2 interaction with active sites. These findings suggest that employing elemental sulfur to produce glycerol-derived porous carbon with sulfur-phosphorus co-doping and subsequent H2O2 treatment is an effective method to produce CO2 capture adsorbents, facilitating the usage of glycerol and elemental sulfur - based products for large-scale applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science of the Total Environment
环境科学-环境科学
CiteScore
17.60
自引率
10.20%
发文量
8726
审稿时长
2.4 months
期刊介绍:
The Science of the Total Environment is an international journal dedicated to scientific research on the environment and its interaction with humanity. It covers a wide range of disciplines and seeks to publish innovative, hypothesis-driven, and impactful research that explores the entire environment, including the atmosphere, lithosphere, hydrosphere, biosphere, and anthroposphere.
The journal's updated Aims & Scope emphasizes the importance of interdisciplinary environmental research with broad impact. Priority is given to studies that advance fundamental understanding and explore the interconnectedness of multiple environmental spheres. Field studies are preferred, while laboratory experiments must demonstrate significant methodological advancements or mechanistic insights with direct relevance to the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


