应用分子内O-to-N磷酸化转移反应设计荧光探针以检测代谢短肽和酰基氨基酸的酶的活性
IF 9.1
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
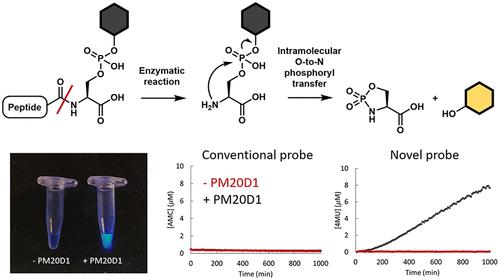
我们提出了利用分子内O-to-N磷酸化转移反应设计蛋白酶/肽酶和酰基氨基酸水解酶荧光探针的策略,其中肽或氨基酸的主链保留在天然底物中,但侧链被设计用于连接荧光基团。该策略可用于设计不倾向于主链修饰和酰基氨基酸水解酶的肽酶/蛋白酶的荧光探针。我们已经开发了GGT5、GGCT和PM20D1的荧光底物,并进行了PM20D1抑制剂/激活剂的筛选,以表征修饰PM20D1活性的化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Application of Intramolecular O-to-N Phosphoryl Transfer Reaction to Design Fluorogenic Probes to Detect Activities of Enzymes That Metabolize Short Peptides and Acylamino Acids
We propose the design strategy of fluorogenic probes of proteases/peptidases and acylamino acid hydrolases utilizing an intramolecular O-to-N phosphoryl transfer reaction, in which the main chain of peptides or amino acids is retained from the natural substrate but the side chain was designed to attach the fluorophore. The strategy is useful to design fluorogenic probes for peptidases/proteases that do not prefer the main chain modification and acylamino acid hydrolases. We have developed the fluorogenic substrates for GGT5, GGCT, and PM20D1 and have performed the screening of PM20D1 inhibitors/activators to characterize the compounds that modify the activity of PM20D1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sensors
Chemical Engineering-Bioengineering
CiteScore
14.50
自引率
3.40%
发文量
372
期刊介绍:
ACS Sensors is a peer-reviewed research journal that focuses on the dissemination of new and original knowledge in the field of sensor science, particularly those that selectively sense chemical or biological species or processes. The journal covers a broad range of topics, including but not limited to biosensors, chemical sensors, gas sensors, intracellular sensors, single molecule sensors, cell chips, and microfluidic devices. It aims to publish articles that address conceptual advances in sensing technology applicable to various types of analytes or application papers that report on the use of existing sensing concepts in new ways or for new analytes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


