还原性非金属的氧化还原活性探测:快速XAS操作研究
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
含铁粘土矿物含有结构铁,具有氧化还原活性,可以参与与水相的电子转移反应。尽管这些氧化还原性质在过去十年中得到了广泛的研究,但在建立稳态氧化还原条件时,动力学和热力学约束各自的作用仍然存在问题。本研究采用快速x射线吸收光谱法(fast - xas)监测了还原后的含铁粘土样品(参考品Nontronite NAu-1)结构中含有的Fe(II)将水中Cr(VI)还原为Cr(III)的动力学。这些测量结果揭示了至少两种反应过程的发生,它们具有不同的快速和慢速动力学速率。根据质量和电子平衡计算,仅位于粘土矿物颗粒边缘的Fe(II)不能解释样品的快速反应性,指出存在从粘土矿物层结构内部向反应位点转移的电子。每次加入Cr(VI)后,黏土结构中的Fe(II)/Fe(III)比值迅速达到稳定状态。这些稳态条件要么与Cr(VI)反应物在Cr(VI)的第一个峰值时完全耗尽相一致,要么与氧化还原对之间的热力学平衡相一致,即在快速反应的Fe(II)池耗尽后,结构Fe(III)/Fe(II)和水相Cr(VI)/Cr(III)之间。这些结果强调需要考虑粘土结构铁氧化还原反应的动力学和热力学控制,以预测环境中氧化还原敏感污染物的命运。本文章由计算机程序翻译,如有差异,请以英文原文为准。
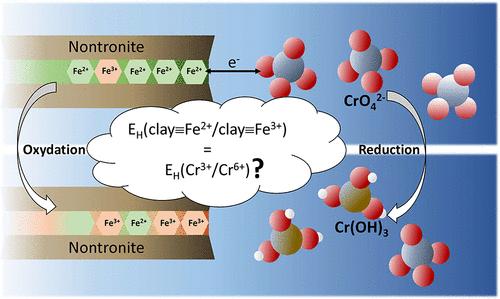
Probing the Redox Reactivity of a Reduced Nontronite: A Quick XAS Operando Study
Fe-bearing clay minerals contain structural iron that can be redox-active and can participate in electron transfer reactions with aqueous species. Although these redox properties have been studied extensively in the past decade, questions remain about the respective roles of kinetic and thermodynamic constraints in establishing steady-state redox conditions. In this study, the reduction kinetics of aqueous Cr(VI) to Cr(III) by Fe(II) contained in the structure of reduced ferruginous clay samples (reference Nontronite NAu-1) was monitored with quick-XAS (X-ray absorption spectroscopy). These measurements revealed the occurrence of at least two reaction processes with contrasting fast and slow kinetic rates. According to mass and electron balance calculations, Fe(II) located at the edge of the clay mineral particles alone cannot account for the fast reactivity of the samples, pointing out the presence of electron transfer from the inner part of the clay mineral layer structure to the reactive sites. The Fe(II)/Fe(III) ratio in the clay structure quickly reached a steady state after each Cr(VI) addition to the solution. These steady-state conditions were consistent with either a complete depletion of the Cr(VI) reactant for the first spikes of Cr(VI) or a thermodynamic equilibrium between the redox couples, i.e., between structural Fe(III)/Fe(II) and aqueous Cr(VI)/Cr(III), after the pool of fast-reacting Fe(II) was depleted. These results highlight the need to consider kinetic and thermodynamic controls of clay structural iron redox reactivity to predict the fate of redox-sensitive contaminants in the environment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


