MIF-ACKR3通过损害癌症恶病质中的脂肪生成导致不可逆的脂肪减少
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
运动和癌症都能导致脂肪组织萎缩。然而,只有癌症相关的体重减轻,即恶病质,以严重的脂肪炎症和纤维化为特征。在这里,我们发现肿瘤分泌的巨噬细胞迁移抑制因子(MIF)是使脂肪干细胞和祖细胞(ASPCs)向促炎和促纤维化方向分化的主要驱动因素,在癌症恶病质中降低脂肪生成能力。相反,运动后循环MIF适度减少。在机制上,非典型趋化因子受体3 (ACKR3)在ASPCs中作为主要的MIF受体介导其病理作用。通过肿瘤细胞中的基因消融或药物阻断抑制MIF,以及aspc特异性Ackr3缺陷,可显著缓解肿瘤诱导的恶病质。这些发现揭示了MIF-ACKR3信号作为肿瘤和恶病质表现之间的关键联系,为癌症恶病质提供了一个有希望的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
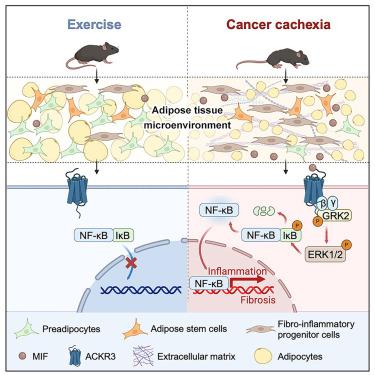
MIF-ACKR3 causes irreversible fat loss by impairing adipogenesis in cancer cachexia
Both exercise and cancer can cause adipose tissue shrinkage. However, only cancer-associated weight loss, namely cachexia, is characterized by profound adipose inflammation and fibrosis. Here, we identified tumor-secreted macrophage migration inhibitory factor (MIF) as a major driver that skews the differentiation of adipose stem and progenitor cells (ASPCs) toward a pro-inflammatory and pro-fibrogenic direction, with reduced adipogenic capacity in cancer cachexia. By contrast, circulating MIF is moderately reduced after exercise. Mechanistically, atypical chemokine receptor 3 (ACKR3) in ASPCs serves as the predominant MIF receptor mediating its pathological effects. Inhibition of MIF by gene ablation in tumor cells or pharmacological blockade, as well as ASPC-specific Ackr3 deficiency, markedly alleviates tumor-induced cachexia. These findings unveil MIF-ACKR3 signaling as a critical link between tumors and cachectic manifestations, providing a promising therapeutic target for cancer cachexia.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


