通过电子势垒电化学系统同时去除Cr(VI), As(III)和磺胺:一个中试研究
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
原位电化学修复是一种很有前途的地下水修复技术。然而,它的应用仅限于短期去污。在这里,我们提出了一种结合电子屏障和黄铁矿的电化学系统,黄铁矿是一种硫化物矿物,能够连续一年完全去除As(III), Cr(VI)和磺胺。我们在两个不同的尺度上评估了由电子屏障和黄铁矿组成的沙盒作为流动电化学反应器:(1)实验室规模的小沙盒,以磺胺作为模型污染物来评估去污性能;(2)中试规模的大沙盒,设计用于同时去除as (III), Cr(VI)和磺胺。小沙箱实现了磺胺100%的去除,证明了联合系统的有效性。在长达一年的时间里,大型沙箱对污染物混合物的去除效率达到100%,即使没有外部中和处理,废水也能保持中性pH值。这种卓越的性能归功于黄铁矿被阳极氧(O₂)激活,产生溶解的铁,导致形成氢氧化铁(例如绿锈),它既是污染物的吸附剂又是沉淀剂。研究结果表明,电化学反应与黄铁矿结合是同时去除有机和无机污染物的有效途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
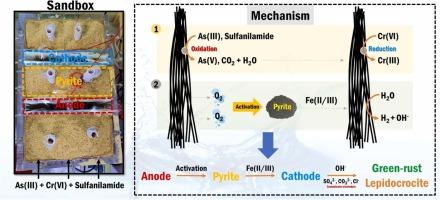
Simultaneous removal of Cr(VI), As(III), and sulfanilamide via an e-barrier electrochemical system: A pilot study
In-situ electrochemical remediation has emerged as a promising groundwater remediation technology. However, its application has been limited to short-term decontamination. Here, we propose an electrochemical system that combines an e-barrier with pyrite, a sulfide mineral capable of completely removing As(III), Cr(VI), and sulfanilamide continuously for one year. We evaluated the sandbox comprising an e-barrier and pyrite as a flow-through electrochemical reactor on two different scales: (1) a lab-scale small sandbox with sulfanilamide as a model contaminant to assess decontamination performance, and (2) a pilot-scale large sandbox designed for the simultaneous removal of As(III), Cr(VI), and sulfanilamide. The small sandbox achieved 100 % removal of sulfanilamide, demonstrating the effectiveness of the combined system. The large sandbox demonstrated 100 % removal efficiency against contaminants mixture for up to one year, with effluent maintaining a neutral pH even without an external neutralizing process. This remarkable performance was attributed to the activation of pyrite by anodic oxygen (O₂), producing dissolved iron that leads to the formation of iron hydroxide (e.g., green rust), which serves both as an adsorbent and precipitant for contaminants. Our findings indicated that the combination of electrochemical reactions and pyrite is an effective approach for the simultaneous removal of organic and inorganic contaminants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


