鼻腔抗cd3单克隆抗体通过il -10依赖性treg -小胶质细胞串扰改善创伤性脑损伤,增强小胶质细胞吞噬,减少神经炎症
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
神经炎症在创伤性脑损伤(TBI)中起着至关重要的作用,有助于损伤和恢复,但目前还没有有效的治疗方法来减轻中枢神经系统(CNS)损伤并促进TBI后的恢复。在本研究中,我们发现鼻腔给药抗cd3单克隆抗体可改善脑挫伤小鼠模型的中枢神经系统损伤和行为缺陷。鼻腔抗cd3诱导产生白细胞介素(IL)-10的调节性T细胞(Treg细胞)迁移到大脑并与小胶质细胞密切接触。Treg细胞直接减少慢性小胶质细胞炎症,并以il -10依赖的方式调节其吞噬功能。在体内整体或特异性地阻断IL-10受体对小胶质细胞的作用,会取消鼻腔抗cd3的有益作用。然而,将产生il -10的Treg细胞过继转移至tbi损伤小鼠,通过增强小胶质细胞吞噬能力和减少小胶质细胞诱导的神经炎症,恢复了这些有益作用。这些发现表明鼻腔抗cd3是治疗TBI和潜在的其他形式急性脑损伤的一种有希望的新治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。

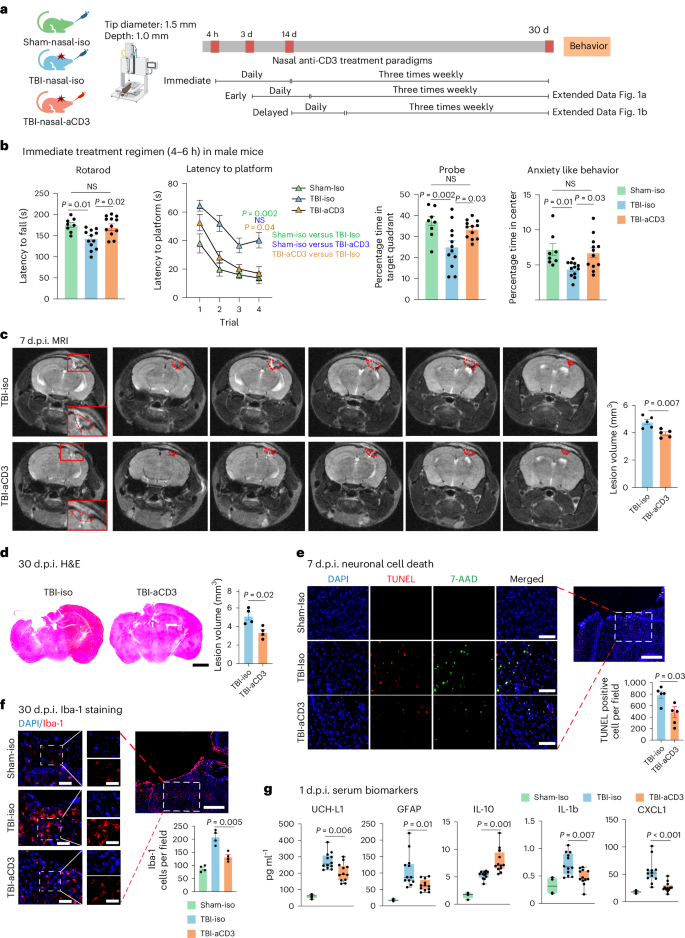
Nasal anti-CD3 monoclonal antibody ameliorates traumatic brain injury, enhances microglial phagocytosis and reduces neuroinflammation via IL-10-dependent Treg–microglia crosstalk
Neuroinflammation plays a crucial role in traumatic brain injury (TBI), contributing to both damage and recovery, yet no effective therapy exists to mitigate central nervous system (CNS) injury and promote recovery after TBI. In the present study, we found that nasal administration of an anti-CD3 monoclonal antibody ameliorated CNS damage and behavioral deficits in a mouse model of contusional TBI. Nasal anti-CD3 induced a population of interleukin (IL)-10-producing regulatory T cells (Treg cells) that migrated to the brain and closely contacted microglia. Treg cells directly reduced chronic microglia inflammation and regulated their phagocytic function in an IL-10-dependent manner. Blocking the IL-10 receptor globally or specifically on microglia in vivo abrogated the beneficial effects of nasal anti-CD3. However, the adoptive transfer of IL-10-producing Treg cells to TBI-injured mice restored these beneficial effects by enhancing microglial phagocytic capacity and reducing microglia-induced neuroinflammation. These findings suggest that nasal anti-CD3 represents a promising new therapeutic approach for treating TBI and potentially other forms of acute brain injury. Nasal anti-CD3 therapy shows promise for treating traumatic brain injury by reducing neuroinflammation and aiding recovery in mice. It induces interleukin-10-producing regulatory T cells that enhance microglial phagocytic activity and reduce chronic inflammation, potentially aiding brain repair.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


