原子转移自由基环化法合成伏诺哌嗪的研究
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文介绍了原子转移自由基环化法(ATRC)合成伏诺哌嗪的新途径。ATRC完成了vonoprazan的关键的1,3,5-三取代吡咯环,随后的芳构化与n -甲胺部分引入环亚胺同时发生,并在吡咯的1位发生磺酰化。此外,我们的方法使vonoprazan的合成达到0.7 kg的规模,而无需在整个过程中分离中间体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
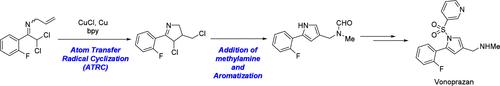
Development of a Synthetic Route to Vonoprazan via Atom Transfer Radical Cyclization
In this Note, a new synthetic route to vonoprazan via atom transfer radical cyclization (ATRC) is described. The pivotal 1,3,5-trisubstituted pyrrole ring of vonoprazan has been accomplished by ATRC, the subsequent aromatization which simultaneously occurred with the introduction of N-methylamine moiety into the cyclic imine, and the sulfonylation at the 1-position of pyrrole. Furthermore, our approach enabled the synthesis of vonoprazan on a 0.7 kg scale without the isolation of intermediates throughout the process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


