利用荧光核碱基替代物进行超分子fret -适体检测和靶位定位
IF 9.1
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
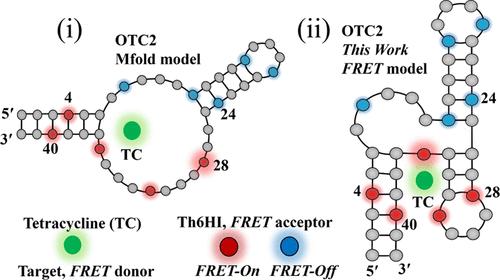
DNA适体能以高亲和力和特异性结合小分子配体,形成独特的超分子结构。获得结合相互作用的结构信息以及敏感的诊断方法是适配体传感器设计的金标准。然而,大多数传感策略提供配体检测而没有结构洞察力,而基于核磁共振或晶体学的结构方法缺乏诊断所需的灵敏度。基于fret的策略可以同时提供这两种方法,特别是在螺旋内空间固定的内部荧光核碱基探针,但双重适体标记可能会损害适体对其目标的亲和力。在此,我们展示了一种基于核碱基替代配体fret的策略,该策略提供了目标位点映射与敏感目标检测相结合,以解决这些挑战。将荧光分子转子(FMR)噻吩查尔酮(Th6HI)核碱基替代物掺入四环素(TC) 42-mer DNA结合适体OTC2中,作为TC供体的受体。时间分辨荧光各向异性实验预测了紧凑的预折叠OTC2适体,几乎不受TC结合的影响。因此,在530 nm处直接激发内部FMR Th6HI对TC结合的响应很小,因为探针刚性没有强烈改变。相比之下,通过TC供体激发在378nm处间接激发Th6HI探针,可提供Th6HI受体的位点依赖敏化荧光(Fsen),与利用固有TC荧光的天然平台相比,可提供更高的TC检测灵敏度。此外,FRET响应提供了靶位映射,为TC-OTC2复合物建立了一个新的结合模型,类似于锤头核酶的三螺旋结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Harnessing a Fluorescent Nucleobase Surrogate for Supramolecular FRET-Aptamer Detection and Target-Site Mapping
DNA aptamers can bind small molecule ligands with high affinity and specificity to produce a unique supramolecular structure. Methods to obtain structural information about the binding interaction coupled with sensitive diagnostics is a gold standard for aptasensor design. However, most sensing strategies afford ligand detection without structural insight, while NMR- or crystallography-based structural methods lack sensitivity required for diagnostics. FRET-based strategies can afford both, especially with internal fluorescent nucleobase probes that are spatially fixed within the helix, but dual aptamer labeling can compromise aptamer affinity toward its target. Herein, we showcase a nucleobase surrogate-ligand FRET-based strategy that affords target-site mapping combined with sensitive target detection that addresses these challenges. A fluorescent molecular rotor (FMR) thiophene chalcone (Th6HI) nucleobase surrogate was incorporated into the tetracycline (TC) 42-mer DNA binding aptamer OTC2 to serve as an acceptor for the TC donor. Time-resolved fluorescence anisotropy experiments predict a compact prefolded OTC2 aptamer that is hardly impacted by TC binding. Consequently, direct excitation of the internal FMR Th6HI at 530 nm affords little response to TC binding, as probe rigidity is not strongly altered. In contrast, indirect excitation of the Th6HI probe through TC donor excitation at 378 nm affords site-dependent sensitized fluorescence (Fsen) of the Th6HI acceptor to afford enhanced sensitivity for TC detection compared to a native platform, which utilizes the intrinsic TC fluorescence. Furthermore, the FRET response provides target-site mapping to build a new binding model for the TC-OTC2 complex that is akin to the three-helical structure of the hammerhead ribozyme.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sensors
Chemical Engineering-Bioengineering
CiteScore
14.50
自引率
3.40%
发文量
372
期刊介绍:
ACS Sensors is a peer-reviewed research journal that focuses on the dissemination of new and original knowledge in the field of sensor science, particularly those that selectively sense chemical or biological species or processes. The journal covers a broad range of topics, including but not limited to biosensors, chemical sensors, gas sensors, intracellular sensors, single molecule sensors, cell chips, and microfluidic devices. It aims to publish articles that address conceptual advances in sensing technology applicable to various types of analytes or application papers that report on the use of existing sensing concepts in new ways or for new analytes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


