线粒体靶向多功能铂基纳米“终端敏感弹丸”提高癌症化疗疗效
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
铂类抗癌药物通过在细胞核DNA (nDNA)内形成加合物、抑制转录和诱导癌细胞凋亡发挥作用。然而,肿瘤细胞已经进化出抵抗这些药物的机制。鉴于线粒体在癌症中的作用以及它们缺乏核苷酸切除修复(NER),靶向线粒体DNA (mtDNA)提供了一种策略。在此基础上,研制了一种铂基末敏弹(TSB),该弹由异功能四价铂前体药作为主战斗部,由三苯基膦(TPP)和二次战斗部FFa(非诺纤维酸)组成制导系统。然后将TSB封装在IR780耦合DSPE-PEG2K中以增强交付(NTSB)。这种设计允许TSB精确靶向肿瘤间线粒体作为其靶向终点,在到达终点后释放游离奥沙利铂(OXA)和FFa。OXA的积累导致与mtDNA交联,导致线粒体功能障碍,而FFa破坏电子传递链(ETC),损害氧化磷酸化(OXPHOS)。此外,在近红外(NIR)照射下,IR780组分产生光疗热效应和活性氧(ROS),从而消耗细胞内谷胱甘肽(GSH)水平,促进Pt与mtDNA交联。体外和体内研究都表明,这种综合方法显著提高了肿瘤细胞对铂类化疗药物的敏感性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
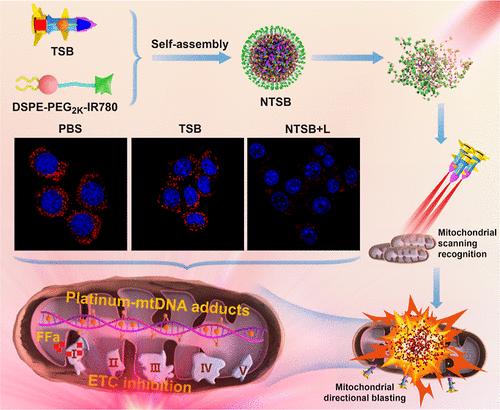
Mitochondrial-Targeted Multifunctional Platinum-Based Nano “Terminal-Sensitive Projectile” for Enhanced Cancer Chemotherapy Efficacy
Platinum-based anticancer drugs exert their effects by forming adducts within nuclear DNA (nDNA), inhibiting transcription and inducing apoptosis in cancer cells. However, tumor cells have evolved mechanisms to resist these drugs. Given mitochondria’s role in cancer and their lack of nucleotide excision repair (NER), targeting mitochondrial DNA (mtDNA) offers a strategy. Herein, a platinum-based terminal-sensitive projectile (TSB) which comprises a heterofunctional tetravalent platinum prodrug as the primary warhead, complemented by a guidance system incorporating triphenylphosphine (TPP) and a secondary warhead, FFa (Fenofibric acid) was developed. TSB was then encapsulated within IR780 coupling DSPE-PEG2K for enhanced delivery (NTSB). This design allows the TSB to be precisely targeted into intertumoral mitochondria as its targeting terminal, releasing free oxaliplatin (OXA) and FFa upon reaching its terminal destination. The accumulation of OXA leads to cross-linking with mtDNA, causing mitochondrial dysfunction, while FFa disrupts the electron transport chain (ETC), impairing oxidative phosphorylation (OXPHOS). Furthermore, under near-infrared (NIR) irradiation, the IR780 component generates a phototherapeutic thermal effect and reactive oxygen species (ROS), which deplete intracellular glutathione (GSH) levels and facilitate Pt cross-linking with mtDNA. Both in vitro and in vivo studies have demonstrated that this comprehensive approach significantly enhances the sensitivity of tumor cells to platinum-based chemotherapeutic drugs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


