水杨基羟肟酸-三辛基甲基铵萃取页岩渗滤液中钒的优化研究
IF 10
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
从含钒页岩中提取钒通常需要用硫酸直接浸出,从而产生含有多种杂质元素的高酸性浸出液,这使得V的提取过程变得复杂。离子液体(IL)萃取方法具有挑战性,因为在浸出溶液中存在阳离子形式的V,而离子液体通常用作阴离子交换萃取剂。在本研究中,提出了STOMAC体系(水杨基羟肟酸(C7H7NO3)与V的摩尔比为1)作为从该复合渗滤液中提取V的创新方案。考察了影响提取效率的关键参数,包括改性剂的选择、C7H7NO3浓度、溶液pH、相比和反应时间。实验结果表明,STOMAC具有良好的提取性能,在最佳条件下,通过三级逆流提取,V的提取率约为99.29%。值得注意的是,铝(Al)、镁(Mg)、铁(Fe)和磷(P)等主要杂质的共萃取率保持在5%以下。通过傅里叶变换红外光谱(FT-IR)、质子核磁共振波谱(1H NMR)和电喷雾电离(ESI)分析进一步阐明V的提取机理和STOMAC的制备。结果表明,水杨基羟基上的氢原子与TOMAC以1:1的比例配位,而羟基与V离子形成1:1的配位。这些结果表明,STOMAC萃取系统可以有效地从酸性渗滤液中回收V,而无需调整pH值,提供了一个更精简、更经济的萃取过程,减少了对额外化学剂的依赖。本文章由计算机程序翻译,如有差异,请以英文原文为准。
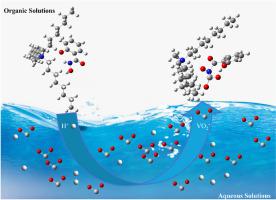
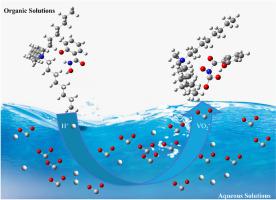
Optimizing vanadium extraction from shale leachate using salicylhydroxamic acid-tri-n-octylmethylammonium
Vanadium (V) extraction from V-bearing shales typically involves direct leaching with sulfuric acid, resulting in highly acidic leachate containing multiple impurity elements, which complicates the V extraction process. Ionic liquid (IL) extraction methods have proven challenging due to the presence of V in its cationic form in the leach solution, while ILs generally function as anion-exchange extractants. In this study, the STOMAC system (salicylhydroxamic acid (C7H7NO3) to V molar ratio of 1) was proposed as an innovative solution for extracting V from this complex leachate. Key parameters affecting the extraction efficiency, including modifier selection, C7H7NO3 concentration, solution pH, phase ratio, and reaction time, were evaluated. Experimental results demonstrated that STOMAC exhibits excellent extraction performance, with approximately 99.29% of V extracted through a three-stage countercurrent extraction process under optimal conditions. Notably, the co-extraction of major impurities such as aluminum (Al), magnesium (Mg), iron (Fe), and phosphorus (P) remained below 5%. The extraction mechanism of V and the preparation of STOMAC were further elucidated through Fourier transform infrared spectroscopy (FT-IR), proton nuclear magnetic resonance spectroscopy (1H NMR), and electrospray ionization (ESI) analyses. The data revealed that the hydrogen (H) atom of the salicylhydroxyl group coordinates with TOMAC in a 1:1 ratio, while the hydroxamic group forms a 1:1 coordination with the V ion. These results suggest that the STOMAC extraction system effectively recovers V from acidic leachate without necessitating pH adjustment, offering a more streamlined and cost-effective extraction process with reduced reliance on additional chemical agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Cleaner Production
环境科学-工程:环境
CiteScore
20.40
自引率
9.00%
发文量
4720
审稿时长
111 days
期刊介绍:
The Journal of Cleaner Production is an international, transdisciplinary journal that addresses and discusses theoretical and practical Cleaner Production, Environmental, and Sustainability issues. It aims to help societies become more sustainable by focusing on the concept of 'Cleaner Production', which aims at preventing waste production and increasing efficiencies in energy, water, resources, and human capital use. The journal serves as a platform for corporations, governments, education institutions, regions, and societies to engage in discussions and research related to Cleaner Production, environmental, and sustainability practices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


