大鼠戊型肝炎病毒作为不明原因急性肝炎的病原
IF 26.8
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
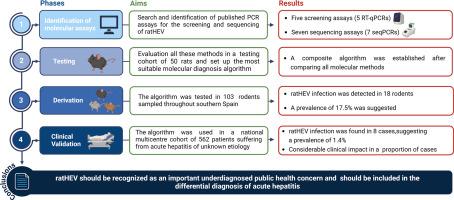
背景和目的戊型肝炎病毒(肝炎病毒)是世界范围内急性肝炎的新病因。非艾滋病毒病例数量有限可能与缺乏适当的分子诊断方法有关;因此,该病毒作为急性肝炎病因的临床影响和广度仍不确定。方法本研究分四个阶段进行。1)鉴定:通过文献检索对分子测定进行鉴定。II)检测:在啮齿类动物测试队列中对方法进行评估,建立最适合的分子诊断算法。III)推导:建立的算法在更大的啮齿动物队列中进行了测试。IV)临床验证:将该算法应用于一组病因不明的急性肝炎患者,从而确定了ratHEV作为急性肝炎病因的频率及其临床影响。结果我们发现在评估的试验中阳性结果的频率存在差异。在比较了所有可用的分子诊断方法后,我们建立了一种分子诊断算法,结果表明,验证队列中103只啮齿动物中有17.5%感染了ratHEV。在临床验证队列中,562例不明原因急性肝炎患者中,在研究的三年中发现了8例ratHEV感染,发生率为1.4%。重度急性肝炎1例(37.5%);4例(50.0%)患者需要住院治疗,其中1例(12.5%)死亡。在这些患者中检测到的菌株与在西班牙大鼠中发现的菌株有密切的系统发育关系。结论我们的研究表明,乙型肝炎病毒是一种新出现的、未被充分诊断的急性肝炎病因。结果提供的证据表明,应监测,并列入急性肝炎的鉴别诊断。影响和意义大鼠戊型肝炎病毒(ratHEV)是一种新型的人畜共患病毒,目前的研究结果表明,分子诊断方法可能不合适。在利用现有的检测方法建立适当的分子诊断算法后,我们证明了ratHEV是一种新兴的、未被诊断的不明原因急性肝炎的病原学因子。该结果还扩大了对欧洲能够感染人类的非hiv毒株多样性的了解。这些发现强烈提示应监测急性肝炎病毒并将其纳入鉴别诊断。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Rat hepatitis E virus as an aetiological agent of acute hepatitis of unknown origin
Background and Aim
Rat hepatitis E virus (ratHEV) is an emerging cause of acute hepatitis worldwide. The limited number of ratHEV cases may be associated with the lack of a proper molecular diagnosis method; thus, the clinical impact and breadth of ratHEV as a cause of acute hepatitis remain uncertain.Methods
The study was carried out in four phases. I) Identification: Molecular assays were identified through a literature search. II) Testing: The methods were evaluated in a rodent testing cohort, and the most suitable molecular diagnosis algorithm was established. III) Derivation: The established algorithm was tested in a larger rodent cohort. IV) Clinical validation: The algorithm was used in a cohort of individuals suffering from acute hepatitis of unknown aetiology, thus establishing the frequency of ratHEV as an aetiological agent of acute hepatitis and its clinical impact.Results
We detected differences in the frequency of positive results among the assays evaluated. After comparing all available molecular methods, we established a molecular diagnostic algorithm, which revealed that 17.5% of the 103 rodents in the validation cohort were infected with ratHEV. In the clinical validation cohort, of 562 patients with acute hepatitis of unknown origin, 8 cases of ratHEV infection were identified during the three years of the study, representing a frequency of 1.4%. One (37.5%) case had severe acute hepatitis; four (50.0%) patients required hospitalization, one of whom (12.5%) died. The strains detected in these patients revealed a close phylogenetic relationship with those found in rats in Spain.Conclusions
Our study demonstrated that ratHEV is an emerging and underdiagnosed cause of acute hepatitis. The results provide evidence that ratHEV should be monitored and included in the differential diagnosis of acute hepatitis.Clinical trial number
ClinicalTrials.gov Identifier: NCT05062967Impact and Implications
While rat hepatitis E virus (ratHEV) is a newly emerging zoonotic virus worldwide, the results of the present study indicate that the molecular diagnosis methods for this virus may be inappropriate. After establishing a proper molecular diagnostic algorithm using available assays, we demonstrated that ratHEV is an emerging and underdiagnosed aetiological agent of acute hepatitis of unknown origin. The results also expand the knowledge of the diversity of ratHEV strains capable of infecting humans in Europe. These findings strongly suggest that ratHEV should be monitored and included in the differential diagnosis of acute hepatitis.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


