镍催化烯烃的还原性芳基烷基化:5-外显子环化vs 6-内环化vs 1,2-芳基向6-内环产物的迁移
IF 3.3
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
采用离散傅里叶变换(DFT)计算系统地研究了镍催化非活化烯烃与芳基溴还原芳基烷基化合成苯融合5-外外和6-内环化合物的详细机理。研究结果表明,在以Zn为还原剂的Ni/biOx体系催化下,含有末端烯烃单元的溴苯优先发生传统的Heck环化和交叉偶联反应,有利于生成5-外显子环化产物。相反,当Zn不存在时,niiii -烷基起关键作用,促进了罕见的1,2-芳基迁移,然后是h原子的萃取,选择性地产生6-内环产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
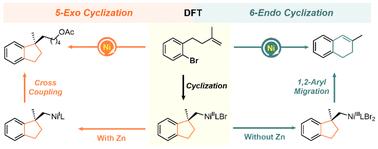
Nickel-catalyzed reductive arylalkylation of alkenes: 5-exo cyclization vs. 6-endo cyclization vs. 1,2-aryl migration to 6-endo product†
The detailed mechanisms of Ni-catalyzed reductive arylalkylation of unactivated alkenes with aryl bromides to synthesize benzene-fused 5-exo and 6-endo cyclic compounds were systematically investigated by DFT calculations. Our finding reveals that, under the catalysis of a Ni/biOx system with Zn as a reductant, bromobenzene containing a terminal olefin unit preferentially undergoes traditional Heck cyclization and cross-coupling reactions, favoring the formation of 5-exo cyclization products. In contrast, when Zn is absent, NiIII-alkyl species play a pivotal role, facilitating a rare 1,2-aryl migration followed by H-atom abstration, which selectively yields 6-endo cyclization products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


