AKR1D1通过促进胆汁酸代谢介导的NK细胞毒性抑制肝癌进展
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
胆汁酸代谢和抗肿瘤免疫在肝癌进展过程中都被破坏。然而,它们之间复杂的监管关系在很大程度上仍不清楚。在这里,我们发现醛酮还原酶1D1 (AKR1D1)的缺失通过肠道微生物组失调促进异石胆酸(isolca)的积累,从而损害自然杀伤细胞(NK)的细胞毒性功能,导致肝细胞癌(HCC)的加速发展。从机制上说,AKR1D1缺乏导致卵形拟杆菌(B. ovatus)比例增加,卵形拟杆菌将鹅脱氧胆酸(CDCA)分解为isolca。此外,积累的iso-LCA以磷酸化- creb1 (p-CREB1)依赖的方式损害肝NK细胞的抗肿瘤活性。保钾利尿剂螺内酯治疗通过靶向isolca介导的肿瘤免疫逃逸,显著增强抗pd1抗体对HCC进展的抑制作用。综上所述,我们的研究结果揭示了AKR1D1与HCC之间以前未被认识到的联系,并表明靶向卵形芽胞杆菌产生的iso-LCA可能是激活NK细胞毒性治疗HCC的一种有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
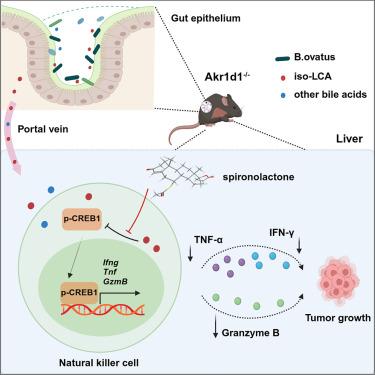
AKR1D1 suppresses liver cancer progression by promoting bile acid metabolism-mediated NK cell cytotoxicity
Bile acid metabolism and antitumor immunity are both disrupted during liver cancer progression. However, the complex regulatory relationship between them remains largely unclear. Here, we find that loss of aldo-keto reductase 1D1 (AKR1D1) promotes the accumulation of isolithocholic acid (iso-LCA) through gut microbiome dysregulation, thereby impairing the cytotoxic function of natural killer (NK) cells and leading to the accelerated development of hepatocellular carcinoma (HCC). Mechanistically, AKR1D1 deficiency leads to an increased proportion of Bacteroidetes ovatus (B. ovatus), which breaks down chenodeoxycholic acid (CDCA) into iso-LCA. Moreover, accumulated iso-LCA impairs the antitumor activity of hepatic NK cells in a phosphorylated-CREB1 (p-CREB1)-dependent manner. The potassium-sparing diuretic spironolactone treatment significantly enhances the inhibitory effect of anti-PD1 antibody on HCC progression by targeting iso-LCA-mediated tumor immune escape. Taken together, our results uncover a previously unappreciated link between AKR1D1 and HCC and suggest that targeting iso-LCA produced by B. ovatus might be a promising strategy to activate NK cell cytotoxicity to treat HCC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


