五聚体基蛋白质纳米管的动态组装
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
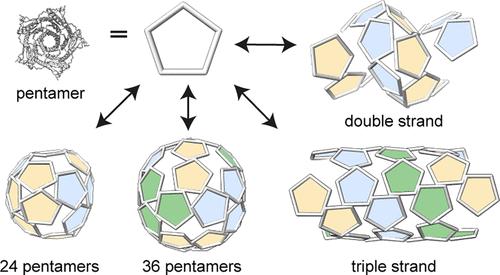
中空的蛋白质颗粒是有用的纳米容器,用于输送和催化。了解多态蛋白组装背后的分子机制和几何理论为设计具有理想形态的蛋白质组装提供了基础。因此,我们发现笼形形成酶Aquifex aeolicus lumazine合成酶(Aquifex aeolicus lumazine synthase, cpAaLS)的圆形排列变体可以根据离子强度的变化组装成各种空心球形和圆柱形结构。低温电子显微镜显示,这些结构完全由五聚体亚基组成,这种剧烈的笼-管转变归因于适度阻碍的3倍对称相互作用和构建块的传递扭转角,其中这两种机制都是由α-螺旋结构域介导的,该结构域通过圆形排列脱离了原始位置。数学模型表明,独特的双链和三链螺旋排列的亚基是最佳的平铺模式,而不同的几何形状应该是可能的通过调节五边形的相互作用角度。这些对动态的、基于五聚体的蛋白质笼和纳米管的结构见解为设计具有定制形态和组装特征的纳米结构提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dynamic Assembly of Pentamer-Based Protein Nanotubes
Hollow proteinaceous particles are useful nanometric containers for delivery and catalysis. Understanding the molecular mechanisms and the geometrical theory behind the polymorphic protein assemblies provides a basis for designing ones with the desired morphology. As such, we found that a circularly permuted variant of a cage-forming enzyme, Aquifex aeolicus lumazine synthase, cpAaLS, assembles into a variety of hollow spherical and cylindrical structures in response to changes in ionic strength. Cryogenic electron microscopy revealed that these structures are composed entirely of pentameric subunits, and the dramatic cage-to-tube transformation is attributed to the moderately hindered 3-fold symmetry interaction and the imparted torsion angle of the building blocks, where both mechanisms are mediated by an α-helix domain that is untethered from the native position by circular permutation. Mathematical modeling suggests that the unique double- and triple-stranded helical arrangements of subunits are optimal tiling patterns, while different geometries should be possible by modulating the interaction angles of the pentagons. These structural insights into dynamic, pentamer-based protein cages and nanotubes afford guidelines for designing nanoarchitectures with customized morphology and assembly characteristics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


