细菌利用病毒休眠来建立CRISPR-Cas免疫
IF 20.6
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
CRISPR-Cas系统通过记录被称为“间隔”的基于dna的感染免疫记忆,为原核生物提供针对外来遗传元素(包括噬菌体)的适应性免疫。没有预先存在免疫力的细胞如何在快速溶解性感染中存活足够长的时间以获得新的间隔并利用它进行防御仍然是一个谜。在这里,我们发现细菌利用温带噬菌体的替代性休眠或“溶原”生命周期来建立CRISPR-Cas免疫。在噬菌体感染期间,进入溶原期的细胞的免疫接种率明显高于裂解期的细胞。此外,在没有外来威胁的情况下,细菌可以获得靶向位于染色体内的噬菌体的间隔物。在这种情况下,Cas9的自我靶向促进了噬菌体的固化,使免疫细胞避免自身免疫。在溶原性的建立和维持过程中,间隔物的优先获取可能解释了为什么天然细菌分离物中的大多数间隔物靶向温带噬菌体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
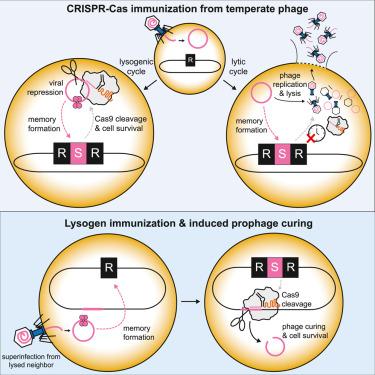
Bacteria exploit viral dormancy to establish CRISPR-Cas immunity
CRISPR-Cas systems provide prokaryotes with adaptive immunity against foreign genetic elements, including bacteriophages, by recording DNA-based immunological memories of infection called “spacers.” How cells without preexisting immunity survive a rapid lytic infection long enough to acquire a new spacer and utilize it for defense remains a mystery. Here, we show that bacteria exploit the alternative dormant or “lysogenic” life cycle of temperate phages to establish CRISPR-Cas immunity. During a phage infection, immunization rates are significantly enhanced in cells entering lysogeny compared to those undergoing lysis. Furthermore, in the absence of a foreign threat, bacteria can acquire spacers targeting prophages residing within the chromosome. In this case, self-targeting by Cas9 promotes curing of the prophage, allowing immunized cells to avoid autoimmunity. The preferred acquisition of spacers during the establishment and maintenance of lysogeny may explain why most spacers in natural bacterial isolates target temperate phages.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


