蛋白质和酶中局部场的理论处理方法
IF 55.8
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
蛋白质支架产生的电场在酶催化中起着至关重要的作用。本文综述了检测、分析和比较电场、静电势及其对酶活性位点内电荷密度影响的理论方法。开创性的方法,如经验价键方法依赖于评估受场影响的离子和共价共振形式。采用极化力场的策略也有助于磁场探测。振动斯塔克效应将计算模拟与实验斯塔克光谱学联系起来,使直接比较成为可能。我们强调了蛋白质动力学如何诱导局部场的波动,影响酶的活性。最近的技术评估了整个活性位点的电场,而不仅仅是特定键的电场,机器学习有助于将这些全局电场与反应性联系起来。分子中原子的量子理论捕获了整个电子密度景观,为场驱动催化提供了化学直观的视角。总的来说,这些方法表明蛋白质产生的领域是高度动态和异质性的,理解这两个方面对于阐明酶的机制至关重要。这种整体的观点通过调整电场赋予合理的酶工程,在药物设计,生物催化和工业应用中有希望的新途径。未来的发展方向包括将电场作为明确的设计目标,以提高催化性能和生化功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
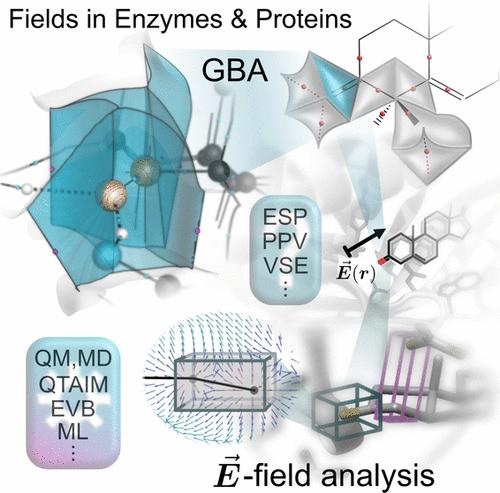
Methods for Theoretical Treatment of Local Fields in Proteins and Enzymes
Electric fields generated by protein scaffolds are crucial in enzymatic catalysis. This review surveys theoretical approaches for detecting, analyzing, and comparing electric fields, electrostatic potentials, and their effects on the charge density within enzyme active sites. Pioneering methods like the empirical valence bond approach rely on evaluating ionic and covalent resonance forms influenced by the field. Strategies employing polarizable force fields also facilitate field detection. The vibrational Stark effect connects computational simulations to experimental Stark spectroscopy, enabling direct comparisons. We highlight how protein dynamics induce fluctuations in local fields, influencing enzyme activity. Recent techniques assess electric fields throughout the active site volume rather than only at specific bonds, and machine learning helps relate these global fields to reactivity. Quantum theory of atoms in molecules captures the entire electron density landscape, providing a chemically intuitive perspective on field-driven catalysis. Overall, these methodologies show protein-generated fields are highly dynamic and heterogeneous, and understanding both aspects is critical for elucidating enzyme mechanisms. This holistic view empowers rational enzyme engineering by tuning electric fields, promising new avenues in drug design, biocatalysis, and industrial applications. Future directions include incorporating electric fields as explicit design targets to enhance catalytic performance and biochemical functionalities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Reviews
化学-化学综合
CiteScore
106.00
自引率
1.10%
发文量
278
审稿时长
4.3 months
期刊介绍:
Chemical Reviews is a highly regarded and highest-ranked journal covering the general topic of chemistry. Its mission is to provide comprehensive, authoritative, critical, and readable reviews of important recent research in organic, inorganic, physical, analytical, theoretical, and biological chemistry.
Since 1985, Chemical Reviews has also published periodic thematic issues that focus on a single theme or direction of emerging research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


