Azirinyl乙基酮的合成及其在C(O)C≡cr取代的nh -吡咯/ nh -咪唑和Azole-Azine / Azole-Azole杂环化合物中的应用
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
采用锂化乙炔与2h -azirine-2-羰基氯化物反应,首次合成了azirinyl取代共轭炔酮。只含一个C(O)C的氮基乙基酮≡在氮环2位的CR取代基是得到α-C (O)C≡CR取代的nh -吡咯和5-[C(O)C≡CR]取代的nh -咪唑的良好前体,它们分别只在氮嘧啶部分选择性地与1,3-二酮和芳基胺反应。各种n -杂环杂环化合物(吡咯-嘧啶,咪唑-嘧啶,吡咯-异恶唑,咪唑-异恶唑,嘧啶-吡咯-噻吩,吡咯-嘧啶-噻吩,咪唑-嘧啶-噻吩,异恶唑-吡咯-噻吩)基于氮嘧啶和C(O)C≡氮基乙基酮的CR基团的正交或多米诺反应制备。本文章由计算机程序翻译,如有差异,请以英文原文为准。
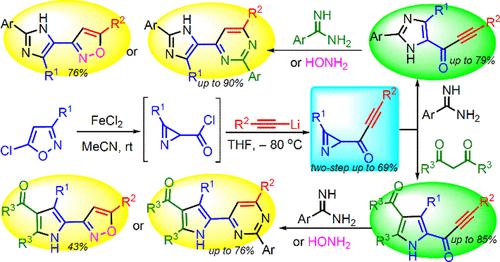
Synthesis of Azirinyl Ethynyl Ketones and Their Use in the Preparation C(O)C≡CR-Substituted NH-Pyrroles/NH-Imidazoles and Azole–Azine/Azole–Azole Heterocyclic Hybrids
Azirinyl-substituted conjugated ynones were first synthesized by the reaction of lithiated acetylenes with 2H-azirine-2-carbonyl chlorides. Azirinyl ethynyl ketones containing only a C(O)C≡CR substituent at position 2 of the azirine ring are good precursors for obtaining α–C(O)C≡CR substituted NH-pyrroles and 5-[C(O)C≡CR]-substituted NH-imidazoles, reacting selectively only at the azirine moiety with 1,3-diketones and arylamidines, respectively. Various N-heterocyclic hybrids (pyrrole-pyrimidine, imidazole-pyrimidine, pyrrole-isoxazole, imidazole-isoxazole, pyrimidine-pyrrole-thiophene, pyrrole-pyrimidine-thiophene, imidazole-pyrimidine-thiophene, isoxazole-pyrrole-thiophene) have been prepared based on orthogonal or domino reactions of an azirine and C(O)C≡CR moieties of azirinyl ethynyl ketones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


