fecl3催化自由基C-N偶联合成吲哚-熔融八元氮杂环及其光物理研究
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报道了一种通过自由基途径构建吲哚-八元杂环的新策略。我们的方法是在温和条件下使用DDQ作为氧化剂,通过fecl3催化的交叉脱氢双C-N键形成串联自由基环化。EPR实验和1H核磁共振时程研究证实,DDQ的加入触发了吡唑n自由基的形成,从而形成了产率较高的吲哚-熔融八元骨架。探讨了合成化合物的结构多样性和合成用途,以及合成化合物的光物理性质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
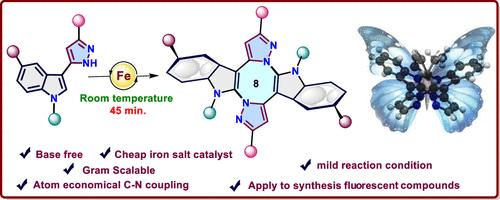
Unveiling the Synthesis of Indole-Fused Eight-Membered Aza-Heterocycles via FeCl3–Catalyzed Radical C–N Coupling and Photophysical Studies
A novel strategy toward construction of indole-fused eight-membered heterocyclic rings through a radical pathway has been reported. Our approach involves tandem radical cyclization via FeCl3-catalyzed cross-dehydrogenative double C–N bond formation using DDQ as an oxidant under mild condition. An EPR experiment and time course 1H NMR study confirm that the addition of DDQ triggers the formation of pyrazole N-radical, which contributes to the formation of an indole-fused eight-member framework with a good yield. The structural diversity and synthetic utility have been explored, along with photophysical properties of the synthesized compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


