酿酒酵母菌非圆锥形氨基酸高效结合的外源和内源优化
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
大多数具有精确非规范氨基酸修饰的治疗性蛋白质和酶只能在真核系统中表达,这将酿酒酵母定位为有前途的底盘细胞。然而,ncAA在酿酒葡萄球菌中的整合受到低效率和复杂转录调控的阻碍。在本研究中,我们通过将tRNALeu CUA拷贝数从1倍增加到2倍,将OMeY浓度从1 mM提高到5 mM,并将培养时间从48 h减少到24 h来优化外源因素。该优化使EGFP40UAG(Tyr40OMeY)的荧光强度(FI)从野生型EGFP的3.17 %提高到105.82 %。通过对synV基因组中发生的结构变异(SVs)进行分析,我们鉴定出了两株具有更高OMeY整合效率的菌株yWJR104和yWJR105。这些菌株的FIs分别达到野生型EGFP的129.52 %和125 %,分别比亲本菌株高2.21倍和2.13倍。值得注意的是,该优化揭示了两个新的基因组靶点YEL014C和YEL013W,它们的缺失导致野生型EGFP的FIs分别为114.52 %和137.6 %。转录组学分析表明,YEL014C的缺失可以促进[PSI+]朊病毒的形成,从而进一步提高ncAA的掺入效率。该研究揭示了酿酒酵母ncAA的调控机制,为优化ncAA在酵母中的掺入开辟了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
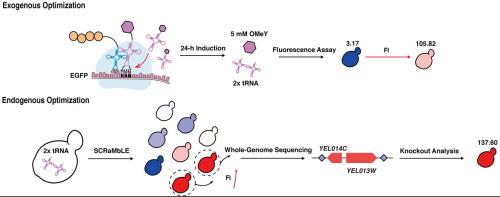
Exogenous and endogenous optimization for efficient nonconical amino acids incorporation in Saccharomyces cerevisiae
Most therapeutic proteins and enzymes with precise noncanonical amino acids (ncAAs) modifications can only be expressed in eukaryotic systems, positioning Saccharomyces cerevisiae as a promising chassis cell. However, ncAA incorporation in S. cerevisiae is hindered by low efficiency and complex transcriptional regulation. In this study, we optimized exogenous factors by increasing the tRNALeu CUA copy from 1x to 2x and raising the OMeY concentration from 1 mM to 5 mM while reducing the cultivation time from 48 h to 24 h. This optimization enhanced the fluorescence intensity (FI) of EGFP40UAG(Tyr40OMeY) from 3.17 % to 105.82 % of wild-type EGFP. Using SCRaMbLE-induced structural variations (SVs) in the synV genome, we identified two strains, yWJR104 and yWJR105, with improved OMeY incorporation efficiencies. These strains achieved FIs of 129.52 % and 125 % of wild-type EGFP, respectively, corresponding to 2.21-fold and 2.13-fold increases relative to the parental strain. Notably, this optimization revealed two novel genomic targets, YEL014C and YEL013W, whose deletion led to FIs of 114.52 % and 137.6 % of wild-type EGFP, respectively. Transcriptomic analysis suggested that deletion of YEL014C may enhance [PSI+] prion formation, which further improved ncAA incorporation efficiency. This study provides insights into ncAA regulatory mechanisms of S. cerevisiae, opening new avenues for optimizing ncAA incorporation in yeast.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


