纳米tio2在岱海中的聚集沉降动力学:pH、天然有机质和离子的相互作用能影响机制
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
通过测定zeta电位、水动力直径(HDD)和A/A0,研究了广泛应用的纳米tio2颗粒在不同腐殖酸(HA)、阳离子(Na+、Ca2+、Mg2+)和pH值的模拟溶液中的聚集沉降。值得注意的是,采用扩展的Derjaguin Landau Verwey Overbeek (XDLVO)理论计算了纳米tio2粒子之间的相互作用能,揭示了各种因素对聚集沉降的机理影响。此外,通过测定纳米tio2在岱海天然水中的聚集沉降情况,验证XDLVO理论在实际水体中的适用性。结果表明,透明质酸通过位阻抑制纳米tio2在Na+溶液中的沉积,同时通过阳离子桥接诱导纳米tio2在Mg2+或Ca2+溶液中的快速聚集。pH值的变化对纳米tio2在含阳离子和HA的溶液中的沉降性能影响不大。此外,纳米tio2在岱海水样中更稳定,A/A0范围为0.83 ~ 0.88。溶解有机碳(DOC)和Na+对纳米tio2的沉降性能影响较大。这些发现对于评价纳米粒子在天然水中的行为具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
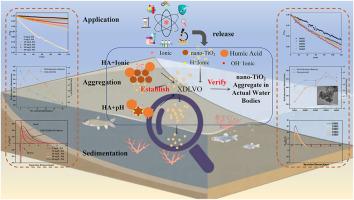
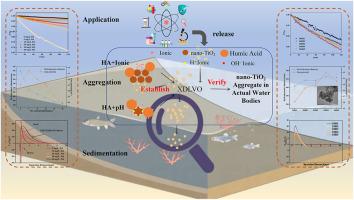
Aggregation and sedimentation kinetics of nano-TiO2 in Daihai Lake: Mechanisms influenced by pH, natural organic matter, and ions based on interaction energy
The widely used nano-TiO2 particles were selected to investigate the aggregation and sedimentation in simulated solutions with different humic acid (HA), cations (Na+, Ca2+, Mg2+) and pH value by measuring zeta potential, hydrodynamic diameter (HDD) and A/A0. Notably, the extended Derjaguin Landau Verwey Overbeek (XDLVO) theory was used to calculate the interaction energy between nano-TiO2 particles to reveal the influence of various factors on aggregation and sedimentation mechanistically. Additionally, the aggregation and sedimentation of nano-TiO2 in natural water from Daihai Lake were determined to verify the applicability of the XDLVO theory in actual water. Results showed that HA inhibits the sedimentation of nano-TiO2 in Na + solution by steric hindrance, while induces rapid aggregation in Mg2+ or Ca2+ solutions due to cation bridging. Change of pH value had little effect on sedimentation performance of nano-TiO2 in solution contain cations and HA. Furthermore, nano-TiO2 were more stable in water sample of Daihai Lake with the A/A0 range from 0.83 to 0.88. And the poor sedimentation performance of nano-TiO2 is result from influence of dissolved organic carbon (DOC) and Na+. These findings are important for evaluating the behavior of nanoparticles in natural water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


