神经元多不饱和脂肪酸对ALS/FTD具有保护作用
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
在这里,我们报告了在肌萎缩性侧索硬化症和额颞叶痴呆(ALS/FTD)最常见的遗传原因C9orf72重复扩增的果蝇模型和人类死后ALS脊髓中脂肪酸和脂质代谢基因表达减少的保守转录组学特征。我们对C9 ALS/FTD果蝇、诱导多能干细胞(iPS)神经元和死后FTD脑组织进行了脂质组学研究。这揭示了含有多不饱和脂肪酸(PUFAs)的磷脂种类的共同和特异性减少。喂食C9 ALS/FTD果蝇PUFAs可适度提高存活率。然而,通过过度表达脂肪酸去饱和酶,增加C9 ALS/FTD果蝇神经元中PUFA水平,导致寿命大幅延长。在C9和TDP-43 ALS/FTD患者的iPS细胞神经元中,脂肪酸去饱和酶的神经元过表达也抑制了应激源诱导的神经元死亡。这些数据暗示神经元脂肪酸饱和与ALS/FTD的发病机制有关,并提示增加神经元PUFA水平的干预可能是有益的。本文章由计算机程序翻译,如有差异,请以英文原文为准。

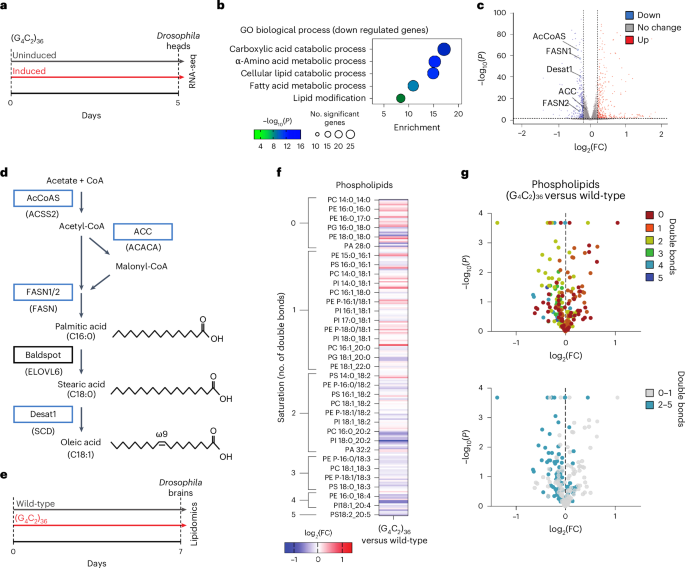
Neuronal polyunsaturated fatty acids are protective in ALS/FTD
Here we report a conserved transcriptomic signature of reduced fatty acid and lipid metabolism gene expression in a Drosophila model of C9orf72 repeat expansion, the most common genetic cause of amyotrophic lateral sclerosis and frontotemporal dementia (ALS/FTD), and in human postmortem ALS spinal cord. We performed lipidomics on C9 ALS/FTD Drosophila, induced pluripotent stem (iPS) cell neurons and postmortem FTD brain tissue. This revealed a common and specific reduction in phospholipid species containing polyunsaturated fatty acids (PUFAs). Feeding C9 ALS/FTD flies PUFAs yielded a modest increase in survival. However, increasing PUFA levels specifically in neurons of C9 ALS/FTD flies, by overexpressing fatty acid desaturase enzymes, led to a substantial extension of lifespan. Neuronal overexpression of fatty acid desaturases also suppressed stressor-induced neuronal death in iPS cell neurons of patients with both C9 and TDP-43 ALS/FTD. These data implicate neuronal fatty acid saturation in the pathogenesis of ALS/FTD and suggest that interventions to increase neuronal PUFA levels may be beneficial. Lipidomics revealed that neurons of patients with ALS/FTD have reduced levels of polyunsaturated fatty acid (PUFA)-containing phospholipids. Increasing neuronal PUFA levels increased survival of Drosophila models of ALS/FTD and patient neurons, suggesting that interventions that increase neuronal PUFA levels in patients with ALS/FTD may also be beneficial.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


