深层共晶溶剂离子液体电解质中多层MXene电极的超级电容器应用
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
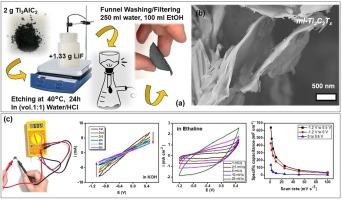
大量研究表明,二维碳化钛(Ti3C2Tx)是MXene家族成员,具有很好的电化学储能特性。Ti3C2Tx由A层在MAX相中选择性蚀刻而成,具有高表面积、亲水表面、层间间距可变、导电性强等特点,适用于各种应用。它的超级电容器性能受到关注,因为它有可能将高功率电容器与高能电池连接起来。本研究比较了多层(ml-) Ti3C2Tx在非水离子液体电解质(Ethaline)、碱性电解质(KOH)和中性电解质(Na2SO4)中的电位窗口和电容性能。采用LiF + HCl刻蚀法制备ml-Ti3C2Tx,并在正(0 ~ +0.6 V)、负(0 ~ -1.2 V)、正负组合(+0.5 ~ -1.2 V)三个电位窗口中进行了测试。在Ethaline中,ml-Ti3C2Tx的面电容在窄电位窗口(0.6 V)内为40 mF/cm2,在宽电位窗口(1.7 V)内增大到356 mF/cm2,扫描速率为5 mV/s。KOH和Na2SO4水溶液由于降解和活性低,不适合用于1.7 V电位窗口的MXene储能。SEM-EDS、XRD、TEM、FTIR和CV表征了ml-Ti3C2Tx的有效合成、功能化和电化学活性。表面修饰,如O-H和C-O振动带,证明了电解质相互作用对提高性能的重要性。研究表明,离子液体电解质如乙炔可以提高能量储存。该研究通过扩大电势窗口和增强电荷存储过程来推进高性能超级电容器,满足日益增长的高效和可持续能源需求。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Multilayered MXene electrodes in deep eutectic solvent ionic liquid electrolyte for supercapacitor applications
Numerous studies have shown that two-dimensional titanium carbide (Ti3C2Tx), a MXene family member, has promising electrochemical characteristics for energy storage. Ti3C2Tx, made by selective etching of A layers in MAX phases, is suitable to various applications owing to its high surface area, hydrophilic surfaces, variable interlayer spacing, and strong electrical conductivity. Its supercapacitor performance has received attention because it has the potential to bridge high-power capacitors with high-energy batteries. This work compares the potential window and capacitance performance of multilayered (ml-) Ti3C2Tx in non-aqueous ionic liquid electrolyte (Ethaline) to alkaline (KOH) and neutral (Na2SO4) electrolytes. LiF + HCl etching method was used to produce ml-Ti3C2Tx, which was tested in three potential windows: positive (0 to +0.6 V), negative (0 to -1.2 V), and combined positive-negative (+0.5 to -1.2 V). The areal capacitance of ml-Ti3C2Tx in Ethaline was determined as 40 mF/cm2 within a narrow potential window (0.6 V) and increased to 356 mF/cm2 within a wider potential window (1.7 V) at a scan rate of 5 mV/s. Aqueous KOH and Na2SO4 electrolytes were unsuitable for MXene based energy storage with 1.7 V potential window due to degradation and low activity. SEM-EDS, XRD, TEM, FTIR and CV indicated effective synthesis, functionalization, and electrochemical activity of ml-Ti3C2Tx. Surface modifications, such as O-H and C-O vibrational bands, demonstrate the importance of electrolyte interactions in improving performance. It was shown that ionic liquid electrolytes like Ethaline can improve energy storage. This study advances high-performance supercapacitors by widening the potential window and enhancing charge storage processes, meeting the growing demand for efficient and sustainable energy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


