业界对透明质酸酶联合配制生物制药的看法
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。

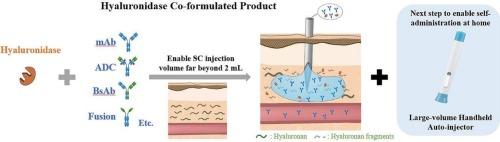
An industry perspective on hyaluronidase co-formulated biopharmaceutics
To deliver biopharmaceutics, subcutaneous (SC) route surpasses intravenous (IV) route unequivocally in time and cost savings, but it has been limited by the injection volume of no greater than 2 mL for a long time. Recently, the adoption of hyaluronidase has become a plausible method to realize high-dose high-volume biopharmaceutical products for SC injection. Among the hyaluronidase family, the recombinant human PH20 appears to be the most reliable candidate with excellent efficacy and safety for co-formulation development. As of 2024, a total of eight hyaluronidase co-formulated biological products have been approved by regulatory authorities. This review article systematically summarized the commercial hyaluronidase co-formulated biopharmaceutics and highlighted the critical aspects of the development of future products regarding selection of hyaluronidase, formulation and process development, non-clinical evaluation, and clinical investigation. Of note, considering the uniqueness of each therapeutic agent, early and effective communication with regulatory authorities is of vital importance to successful development. Discussions were further Expanded to cover the combination of hyaluronidase co-formulations with large-volume handheld autoinjectors. The ultimate goal of this review is to provide a practical and comprehensive reference that will substantially contribute to the development of hyaluronidase co-formulated biopharmaceuticals, thereby advancing the field and benefiting patients worldwide.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


