烯化酮和非烯化酮的C-H胺化。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们提出了一种将α(或β)位置的可烯化和非烯化酮胺化为羰基的方法。这种方法是基于将相应的氰醇转化为碳叠盐,碳叠盐是分子内亚硝基插入到相邻C-H键的热反应的前体。所得氨基甲酸酯在碱性条件下水解,同时羰基再生,生成氨基酮。本文章由计算机程序翻译,如有差异,请以英文原文为准。
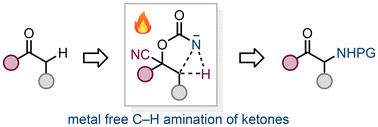
C–H amination of enolizable and nonenolizable ketones†
We present a method for the amination of enolizable and non-enolizable ketones in the alpha (or beta) position to the carbonyl group. This approach is based on the conversion of the corresponding cyanohydrins to carbonazidates, precursors for thermal intramolecular nitrene insertion reactions into the adjacent C–H bond. Hydrolysis of the resulting carbamates under basic conditions with simultaneous regeneration of the carbonyl group yields amino ketones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


