用于OER的可扩展Co-MOF薄膜:通过表面重建实现低过电位和增强催化活性
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
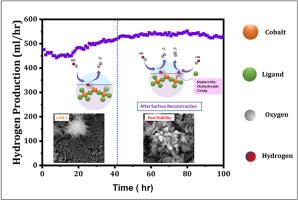
降低成本仍然是绿色氢经济面临的关键挑战,因此需要替换昂贵的RuO2和IrO2催化剂用于析氧反应(OER)。金属有机骨架(mof)具有高表面积、孔隙率和可调特性,是高效、经济的OER的有希望的替代品。本研究采用不同金属浓度的反应扩散法,探索在不锈钢衬底上低成本、无粘结剂合成Co-MOF薄膜的方法。优化后的CoM-2薄膜在10 mA cm -2下的过电位为275 mV, Tafel斜率为76 mV.dec - 1, OER的ECSA为251 cm2,令人印象深刻。FESEM研究发现其独特的茉莉花状簇生长在顶部垂直排列的棒状表面形态,XPS证实其具有较高的吡啶氮含量和中等含量的Co-N,从而提高了其催化效率。无粘结剂的合成方法进一步促进了CoM-2的特殊催化活性。在原型水电解装置中,CoM-2薄膜的析氢速率达到了1244 mL / h,并且在连续运行100小时后,随着产量的增加,表现出了良好的稳定性。这种活性的增强归因于在碱性介质中的表面重建,其中表面暴露的与钴连接的配体被羟基取代,并增加了OER的活性位点数量。这些发现突出了Co-MOF薄膜在可持续能源应用中作为一种高效、经济、可扩展的OER电催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Scalable Co-MOF thin films for OER: Achieving low overpotential and enhanced catalytic activity via surface reconstruction
The cost reduction remains a critical challenge for the green hydrogen economy, necessitating the replacement of expensive RuO2 and IrO2 catalysts for the oxygen evolution reaction (OER). Metal-organic frameworks (MOFs), with their high surface area, porosity, and tunable properties, emerge as promising alternatives for efficient and cost-effective OER. This study uses the Reaction-Diffusion method with varying metal concentrations to explore the cost-effective and binder-free synthesis of Co-MOF thin films on a stainless steel substrate. The optimised CoM-2 thin film demonstrating an overpotential of 275 mV at 10 mA cm−2, a Tafel slope of 76 mV.dec−1 and an impressive ECSA of 251 cm2 for OER. The FESEM study revealed its unique jasmine flower-like clusters grown a top vertically well-aligned rod-like surface morphology, while XPS confirmed the presence of high pyridine-nitrogen content and moderate content of Co–N, which enhances its catalytic efficiency. The binder-free synthesis approach further contributes to the exceptional catalytic activity of CoM-2. In the prototype water electrolysis setup, the CoM-2 thin film achieved a hydrogen evolution rate of 1244 mL per hour and demonstrated good stability with increasing rate of production over 100 h of continuous operation. This enhancement in activity is attributed to surface reconstruction in the alkaline medium, where surface-exposed ligands attached to cobalt were replaced with oxyhydroxide groups and increased the number of active sites for OER. These findings highlight Co-MOF thin film as an efficient, economical, scalable electrocatalyst for OER in sustainable energy applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


